Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeted Therapy of Atherosclerosis by a Broad-Spectrum Reactive Oxygen Species Scavenging Nanoparticle with Intrinsic Anti-inflammatory Activity
ACS Nano ( IF 15.8 ) Pub Date : 2018-08-16 00:00:00 , DOI: 10.1021/acsnano.8b02037 Yuquan Wang 1 , Lanlan Li , Weibo Zhao , Yin Dou , Huijie An , Hui Tao , Xiaoqiu Xu , Yi Jia , Shan Lu , Jianxiang Zhang , Houyuan Hu
ACS Nano ( IF 15.8 ) Pub Date : 2018-08-16 00:00:00 , DOI: 10.1021/acsnano.8b02037 Yuquan Wang 1 , Lanlan Li , Weibo Zhao , Yin Dou , Huijie An , Hui Tao , Xiaoqiu Xu , Yi Jia , Shan Lu , Jianxiang Zhang , Houyuan Hu
Affiliation
|
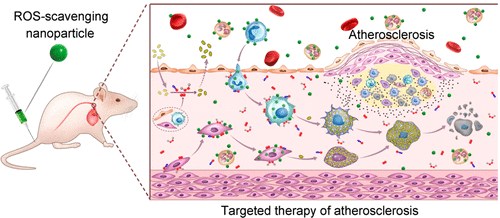
Atherosclerosis is a leading cause of vascular diseases worldwide. Whereas antioxidative therapy has been considered promising for the treatment of atherosclerosis in view of a critical role of reactive oxygen species (ROS) in the pathogenesis of atherosclerosis, currently available antioxidants showed considerably limited clinical outcomes. Herein, we hypothesize that a broad-spectrum ROS-scavenging nanoparticle can serve as an effective therapy for atherosclerosis, taking advantage of its antioxidative stress activity and targeting effects. As a proof of concept, a broad-spectrum ROS-eliminating material was synthesized by covalently conjugating a superoxide dismutase mimetic agent Tempol and a hydrogen-peroxide-eliminating compound of phenylboronic acid pinacol ester onto a cyclic polysaccharide β-cyclodextrin (abbreviated as TPCD). TPCD could be easily processed into a nanoparticle (TPCD NP). The obtained nanotherapy TPCD NP could be efficiently and rapidly internalized by macrophages and vascular smooth muscle cells (VSMCs). TPCD NPs significantly attenuated ROS-induced inflammation and cell apoptosis in macrophages, by eliminating overproduced intracellular ROS. Also, TPCD NPs effectively inhibited foam cell formation in macrophages and VSMCs by decreasing internalization of oxidized low-density lipoprotein. After intravenous (i.v.) administration, TPCD NPs accumulated in atherosclerotic lesions of apolipoprotein E-deficient (ApoE–/–) mice by passive targeting through the dysfunctional endothelium and translocation via inflammatory cells. TPCD NPs significantly inhibited the development of atherosclerosis in ApoE–/– mice after i.v. delivery. More importantly, therapy with TPCD NPs afforded stabilized plaques with less cholesterol crystals, a smaller necrotic core, thicker fibrous cap, and lower macrophages and matrix metalloproteinase-9, compared with those treated with control drugs previously developed for antiatherosclerosis. The therapeutic benefits of TPCD NPs mainly resulted from reduced systemic and local oxidative stress and inflammation as well as decreased inflammatory cell infiltration in atherosclerotic plaques. Preliminary in vivo tests implied that TPCD NPs were safe after long-term treatment via i.v. injection. Consequently, TPCD NPs can be developed as a potential antiatherosclerotic nanotherapy.
中文翻译:

广谱活性氧清除具有内在抗炎活性的纳米粒子的动脉粥样硬化的靶向治疗。
动脉粥样硬化是全世界血管疾病的主要原因。考虑到活性氧(ROS)在动脉粥样硬化发病机理中的关键作用,抗氧化疗法被认为是治疗动脉粥样硬化的有希望的方法,而目前可用的抗氧化剂显示出相当有限的临床结果。在本文中,我们假设利用广谱的ROS清除纳米颗粒可以利用其抗氧化应激活性和靶向作用来作为动脉粥样硬化的有效疗法。作为概念证明,通过将超氧化物歧化酶模拟剂Tempol和苯基硼酸频哪醇酯的过氧化氢消除化合物共价结合到环状多糖β-环糊精(简称TPCD)上,合成了广谱的ROS消除材料。 。TPCD可以轻松加工成纳米颗粒(TPCD NP)。巨噬细胞和血管平滑肌细胞(VSMC)可以高效,快速地将获得的纳米疗法TPCD NP内在化。TPCD NPs通过消除过度产生的细胞内ROS,大大减轻了ROS诱导的炎症和巨噬细胞的细胞凋亡。而且,TPCD NP通过减少氧化的低密度脂蛋白的内在化作用,有效抑制了巨噬细胞和VSMC中泡沫细胞的形成。静脉内(iv)给药后,TPCD NPs积聚在载脂蛋白E缺乏(ApoE)的动脉粥样硬化病变中 TPCD NPs通过消除过度产生的细胞内ROS,大大减轻了ROS诱导的炎症和巨噬细胞的细胞凋亡。而且,TPCD NP通过减少氧化的低密度脂蛋白的内在化作用,有效抑制了巨噬细胞和VSMC中泡沫细胞的形成。静脉内(iv)给药后,TPCD NPs积聚在载脂蛋白E缺乏(ApoE)的动脉粥样硬化病变中 TPCD NPs通过消除过度产生的细胞内ROS,大大减轻了ROS诱导的炎症和巨噬细胞的细胞凋亡。而且,TPCD NP通过减少氧化的低密度脂蛋白的内在化作用,有效抑制了巨噬细胞和VSMC中泡沫细胞的形成。静脉内(iv)给药后,TPCD NPs积聚在载脂蛋白E缺乏(ApoE)的动脉粥样硬化病变中– / –)小鼠通过功能失调的内皮细胞进行被动靶向,并通过炎症细胞进行易位。TPCD NPs在静脉输注后显着抑制了ApoE – / –小鼠的动脉粥样硬化的发展。更重要的是,与先前使用抗动脉粥样硬化对照药物治疗的患者相比,使用TPCD NPs治疗的患者可得到稳定的斑块,其胆固醇晶体更少,坏死核心更小,纤维帽更厚,巨噬细胞和基质金属蛋白酶9更低。TPCD NPs的治疗优势主要来自全身和局部氧化应激和炎症的减少,以及动脉粥样硬化斑块中炎症细胞浸润的减少。体内初步试验表明,经静脉注射长期治疗后,TPCD NPs是安全的。因此,TPCD NPs可以发展为潜在的抗动脉粥样硬化纳米疗法。
更新日期:2018-08-16
中文翻译:

广谱活性氧清除具有内在抗炎活性的纳米粒子的动脉粥样硬化的靶向治疗。
动脉粥样硬化是全世界血管疾病的主要原因。考虑到活性氧(ROS)在动脉粥样硬化发病机理中的关键作用,抗氧化疗法被认为是治疗动脉粥样硬化的有希望的方法,而目前可用的抗氧化剂显示出相当有限的临床结果。在本文中,我们假设利用广谱的ROS清除纳米颗粒可以利用其抗氧化应激活性和靶向作用来作为动脉粥样硬化的有效疗法。作为概念证明,通过将超氧化物歧化酶模拟剂Tempol和苯基硼酸频哪醇酯的过氧化氢消除化合物共价结合到环状多糖β-环糊精(简称TPCD)上,合成了广谱的ROS消除材料。 。TPCD可以轻松加工成纳米颗粒(TPCD NP)。巨噬细胞和血管平滑肌细胞(VSMC)可以高效,快速地将获得的纳米疗法TPCD NP内在化。TPCD NPs通过消除过度产生的细胞内ROS,大大减轻了ROS诱导的炎症和巨噬细胞的细胞凋亡。而且,TPCD NP通过减少氧化的低密度脂蛋白的内在化作用,有效抑制了巨噬细胞和VSMC中泡沫细胞的形成。静脉内(iv)给药后,TPCD NPs积聚在载脂蛋白E缺乏(ApoE)的动脉粥样硬化病变中 TPCD NPs通过消除过度产生的细胞内ROS,大大减轻了ROS诱导的炎症和巨噬细胞的细胞凋亡。而且,TPCD NP通过减少氧化的低密度脂蛋白的内在化作用,有效抑制了巨噬细胞和VSMC中泡沫细胞的形成。静脉内(iv)给药后,TPCD NPs积聚在载脂蛋白E缺乏(ApoE)的动脉粥样硬化病变中 TPCD NPs通过消除过度产生的细胞内ROS,大大减轻了ROS诱导的炎症和巨噬细胞的细胞凋亡。而且,TPCD NP通过减少氧化的低密度脂蛋白的内在化作用,有效抑制了巨噬细胞和VSMC中泡沫细胞的形成。静脉内(iv)给药后,TPCD NPs积聚在载脂蛋白E缺乏(ApoE)的动脉粥样硬化病变中– / –)小鼠通过功能失调的内皮细胞进行被动靶向,并通过炎症细胞进行易位。TPCD NPs在静脉输注后显着抑制了ApoE – / –小鼠的动脉粥样硬化的发展。更重要的是,与先前使用抗动脉粥样硬化对照药物治疗的患者相比,使用TPCD NPs治疗的患者可得到稳定的斑块,其胆固醇晶体更少,坏死核心更小,纤维帽更厚,巨噬细胞和基质金属蛋白酶9更低。TPCD NPs的治疗优势主要来自全身和局部氧化应激和炎症的减少,以及动脉粥样硬化斑块中炎症细胞浸润的减少。体内初步试验表明,经静脉注射长期治疗后,TPCD NPs是安全的。因此,TPCD NPs可以发展为潜在的抗动脉粥样硬化纳米疗法。































 京公网安备 11010802027423号
京公网安备 11010802027423号