当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemo-enzymatic Total Syntheses of Jorunnamycin A, Saframycin A, and N-Fmoc Saframycin Y3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-08-16 , DOI: 10.1021/jacs.8b07161 Ryo Tanifuji 1 , Kento Koketsu 2 , Michiko Takakura 2 , Ryutaro Asano 3 , Atsushi Minami 2 , Hideaki Oikawa 2 , Hiroki Oguri 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-08-16 , DOI: 10.1021/jacs.8b07161 Ryo Tanifuji 1 , Kento Koketsu 2 , Michiko Takakura 2 , Ryutaro Asano 3 , Atsushi Minami 2 , Hideaki Oikawa 2 , Hiroki Oguri 1
Affiliation
|
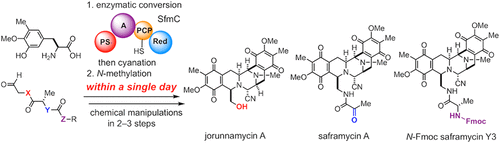
The antitumor tetrahydroisoquinoline (THIQ) alkaloids share a common pentacyclic scaffold that is biosynthesized by nonribosomal peptide synthetases involving unique enzymatic Pictet-Spengler cyclizations. Herein we report concise and divergent chemo-enzymatic total syntheses of THIQ alkaloids by merging precise chemical synthesis with in vitro engineered biosynthesis. A recombinant enzyme SfmC responsible for the biosynthesis of saframycin A was adapted for the assembly of these natural products and their derivatives, by optimizing designer substrates compatible with SfmC through chemical synthesis. The appropriately functionalized pentacyclic skeleton were efficiently synthesized by streamlining the linkage between SfmC-catalyzed multistep enzymatic conversions and chemical manipulations of the intermediates to install aminonitrile and N-methyl groups. This approach allowed rapid access to the elaborated pentacyclic skeleton in a single day starting from two simple synthetic substrates without isolation of the intermediates. Further functional group manipulations allowed operationally simple and expeditious syntheses of jorunnamycin A, saframycin A, and N-Fmoc saframycin Y3 that could be versatile and common precursors for the artificial production of other antitumor THIQ alkaloids and their variants.
中文翻译:

Jorunnamycin A、Saframycin A 和 N-Fmoc Saframycin Y3 的化学酶促总合成
抗肿瘤四氢异喹啉 (THIQ) 生物碱具有共同的五环支架,该支架由非核糖体肽合成酶生物合成,涉及独特的酶促 Pictet-Spengler 环化。在此,我们通过将精确的化学合成与体外工程生物合成相结合,报告了 THIQ 生物碱的简洁和不同的化学酶促全合成。通过化学合成优化与 SfmC 兼容的设计底物,一种负责番红霉素 A 生物合成的重组酶 SfmC 适用于这些天然产物及其衍生物的组装。通过简化 SfmC 催化的多步酶促转化和中间体的化学操作以安装氨基腈和 N-甲基之间的连接,有效地合成了适当官能化的五环骨架。这种方法允许在一天内从两个简单的合成底物开始快速获得精心设计的五环骨架,而无需分离中间体。进一步的官能团操作使得 jorunnamycin A、saframycin A 和 N-Fmoc saframycin Y3 的合成操作简单而迅速,这些合成物可能是用于人工生产其他抗肿瘤 THIQ 生物碱及其变体的通用前体。这种方法允许在一天内从两个简单的合成底物开始快速获得精心设计的五环骨架,而无需分离中间体。进一步的官能团操作使得 jorunnamycin A、saframycin A 和 N-Fmoc saframycin Y3 的合成操作简单而迅速,这些合成物可能是用于人工生产其他抗肿瘤 THIQ 生物碱及其变体的通用前体。这种方法允许在一天内从两个简单的合成底物开始快速获得精心设计的五环骨架,而无需分离中间体。进一步的官能团操作使得 jorunnamycin A、saframycin A 和 N-Fmoc saframycin Y3 的合成操作简单而迅速,这些合成物可能是用于人工生产其他抗肿瘤 THIQ 生物碱及其变体的通用前体。
更新日期:2018-08-16
中文翻译:

Jorunnamycin A、Saframycin A 和 N-Fmoc Saframycin Y3 的化学酶促总合成
抗肿瘤四氢异喹啉 (THIQ) 生物碱具有共同的五环支架,该支架由非核糖体肽合成酶生物合成,涉及独特的酶促 Pictet-Spengler 环化。在此,我们通过将精确的化学合成与体外工程生物合成相结合,报告了 THIQ 生物碱的简洁和不同的化学酶促全合成。通过化学合成优化与 SfmC 兼容的设计底物,一种负责番红霉素 A 生物合成的重组酶 SfmC 适用于这些天然产物及其衍生物的组装。通过简化 SfmC 催化的多步酶促转化和中间体的化学操作以安装氨基腈和 N-甲基之间的连接,有效地合成了适当官能化的五环骨架。这种方法允许在一天内从两个简单的合成底物开始快速获得精心设计的五环骨架,而无需分离中间体。进一步的官能团操作使得 jorunnamycin A、saframycin A 和 N-Fmoc saframycin Y3 的合成操作简单而迅速,这些合成物可能是用于人工生产其他抗肿瘤 THIQ 生物碱及其变体的通用前体。这种方法允许在一天内从两个简单的合成底物开始快速获得精心设计的五环骨架,而无需分离中间体。进一步的官能团操作使得 jorunnamycin A、saframycin A 和 N-Fmoc saframycin Y3 的合成操作简单而迅速,这些合成物可能是用于人工生产其他抗肿瘤 THIQ 生物碱及其变体的通用前体。这种方法允许在一天内从两个简单的合成底物开始快速获得精心设计的五环骨架,而无需分离中间体。进一步的官能团操作使得 jorunnamycin A、saframycin A 和 N-Fmoc saframycin Y3 的合成操作简单而迅速,这些合成物可能是用于人工生产其他抗肿瘤 THIQ 生物碱及其变体的通用前体。















































 京公网安备 11010802027423号
京公网安备 11010802027423号