当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photocatalytic reduction of CO2 to CO and formate by a novel Co(ii) catalyst containing a cis-oxygen atom: photocatalysis and DFT calculations†
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-08-17 00:00:00 , DOI: 10.1039/c8dt02148a Cheng-Yi Zhu 1, 2, 3, 4, 5 , Ya-Qiong Zhang 1, 2, 3, 4, 5 , Rong-Zhen Liao 1, 2, 3, 4, 5 , Wu Xia 1, 2, 3, 4, 5 , Jun-Chao Hu 1, 2, 3, 4, 5 , Jin Wu 1, 2, 3, 4, 5 , Hongfang Liu 1, 2, 3, 4, 5 , Feng Wang 1, 2, 3, 4, 5
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-08-17 00:00:00 , DOI: 10.1039/c8dt02148a Cheng-Yi Zhu 1, 2, 3, 4, 5 , Ya-Qiong Zhang 1, 2, 3, 4, 5 , Rong-Zhen Liao 1, 2, 3, 4, 5 , Wu Xia 1, 2, 3, 4, 5 , Jun-Chao Hu 1, 2, 3, 4, 5 , Jin Wu 1, 2, 3, 4, 5 , Hongfang Liu 1, 2, 3, 4, 5 , Feng Wang 1, 2, 3, 4, 5
Affiliation
|
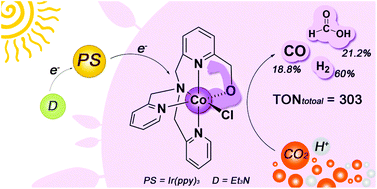
The conversion of carbon dioxide (CO2) to fuels or value-added chemicals by a photocatalytic system has recently been of growing research interest. One of the challenges is the development of new catalysts with high activity and low cost. Cobalt complexes have long been used as catalysts for the reduction of CO2 in either electrochemical or photochemical systems. Recently, a series of cis-CoII complexes of tetradentate pyridine–amine ligands (N4-ligands) exhibited high activity in the reduction of CO2 in homogeneous photocatalytic systems. However, only CO was obtained as the reduction product. In this regard, herein, we report a novel cis-CoII complex C1 supported by an N4 ligand derivatized with TPA (TPA = tris(2-pyridylmethyl)amine). In contrast to the aforementioned CoII catalysts, which contain two halogen atoms at cis-positions, C1 contains one oxygen atom at one cis-coordination site. The structure of C1 was fully characterized by MS, elemental analysis, and single-crystal X-ray diffraction. Experiments on the photocatalytic reduction of CO2 revealed that C1 is able to convert CO2 to not only CO but also formate in a homogeneous system containing C1 as a catalyst, Ir(ppy)3 as a photosensitizer, and triethylamine as an electron donor under visible-light irradiation. The catalytic activity and distribution of reduction products of this system are highly affected by the solvent environment. The presence of water in this system enhances the efficiency of 2H+-to-H2 and CO2-to-formate conversions. Electrochemical and steady-state emission quenching experiments indicate that photoinduced electron transfer from excited Ir(ppy)3 to C1 is thermodynamically feasible. A photogenerated CoI species is suggested to be the active species involved in the reduction of CO2 and protons. DFT calculations were performed to elucidate the catalytic pathways of the formation of CO, formate, and H2 in this system; four pathways, namely, one for the formation of CO, one for the formation of hydrogen, and two for the formation of formate, were suggested. The results revealed that the oxygen atom at the cis-coordination site in C1 plays an important role in stabilizing the transition state during the transformation of CO2 at the cobalt center.
中文翻译:

一种新型的含有顺式氧原子的Co(ii)催化剂 将CO 2光催化还原为CO和甲酸盐:光催化和DFT计算†
近来,通过光催化系统将二氧化碳(CO 2)转化为燃料或增值化学品已引起越来越多的研究兴趣。挑战之一是开发具有高活性和低成本的新型催化剂。钴配合物长期以来一直用作在电化学或光化学体系中还原CO 2的催化剂。最近,四齿吡啶-胺配体(N 4-配体)的一系列顺式-Co II配合物在均相光催化体系中表现出高的还原CO 2活性。但是,仅获得CO作为还原产物。在这方面,本文报道了一种新颖的顺式-Co II复杂C1由N支持4用TPA(TPA =三(2-吡啶基甲基)胺)衍生的配体。与前述的在顺式位置含有两个卤素原子的Co II催化剂相反,C 1在一个顺式配位位置含有一个氧原子。通过MS,元素分析和单晶X射线衍射充分表征了C1的结构。在光催化还原CO的实验2表明,C1能够将CO转化2不仅CO而且甲酸在含有均相体系C1作为催化剂的Ir(ppy)3在可见光照射下作为光敏剂的三乙胺和作为电子给体的三乙胺。该体系的催化活性和还原产物的分布受溶剂环境的影响很大。该系统中水的存在提高了2H + -向H 2转化和CO 2-向甲酸酯转化的效率。电化学和稳态发射猝灭实验表明,从激发的Ir(ppy)3到C1的光诱导电子转移在热力学上是可行的。光生的Co I物种被认为是减少CO 2的活性物种和质子。进行DFT计算以阐明该系统中CO,甲酸和H 2形成的催化途径。提出了四种途径,即一种用于形成CO,一种用于形成氢,以及两种用于形成甲酸的途径。结果表明,在钴中心CO 2转化过程中,C1中顺式配位位点的氧原子在稳定过渡态中起着重要作用。
更新日期:2018-08-17
中文翻译:

一种新型的含有顺式氧原子的Co(ii)催化剂 将CO 2光催化还原为CO和甲酸盐:光催化和DFT计算†
近来,通过光催化系统将二氧化碳(CO 2)转化为燃料或增值化学品已引起越来越多的研究兴趣。挑战之一是开发具有高活性和低成本的新型催化剂。钴配合物长期以来一直用作在电化学或光化学体系中还原CO 2的催化剂。最近,四齿吡啶-胺配体(N 4-配体)的一系列顺式-Co II配合物在均相光催化体系中表现出高的还原CO 2活性。但是,仅获得CO作为还原产物。在这方面,本文报道了一种新颖的顺式-Co II复杂C1由N支持4用TPA(TPA =三(2-吡啶基甲基)胺)衍生的配体。与前述的在顺式位置含有两个卤素原子的Co II催化剂相反,C 1在一个顺式配位位置含有一个氧原子。通过MS,元素分析和单晶X射线衍射充分表征了C1的结构。在光催化还原CO的实验2表明,C1能够将CO转化2不仅CO而且甲酸在含有均相体系C1作为催化剂的Ir(ppy)3在可见光照射下作为光敏剂的三乙胺和作为电子给体的三乙胺。该体系的催化活性和还原产物的分布受溶剂环境的影响很大。该系统中水的存在提高了2H + -向H 2转化和CO 2-向甲酸酯转化的效率。电化学和稳态发射猝灭实验表明,从激发的Ir(ppy)3到C1的光诱导电子转移在热力学上是可行的。光生的Co I物种被认为是减少CO 2的活性物种和质子。进行DFT计算以阐明该系统中CO,甲酸和H 2形成的催化途径。提出了四种途径,即一种用于形成CO,一种用于形成氢,以及两种用于形成甲酸的途径。结果表明,在钴中心CO 2转化过程中,C1中顺式配位位点的氧原子在稳定过渡态中起着重要作用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号