当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Behind the Scenes of Group 4 Metallocene Catalysis: Examination of the Metal–Carbon Bond
Organometallics ( IF 2.5 ) Pub Date : 2018-08-15 , DOI: 10.1021/acs.organomet.8b00339 Martin R. Machat , Andreas Fischer 1 , Dominik Schmitz 1 , Marcel Vöst 1 , Markus Drees , Christian Jandl , Alexander Pöthig , Nicola P. M. Casati 2 , Wolfgang Scherer 1 , Bernhard Rieger
Organometallics ( IF 2.5 ) Pub Date : 2018-08-15 , DOI: 10.1021/acs.organomet.8b00339 Martin R. Machat , Andreas Fischer 1 , Dominik Schmitz 1 , Marcel Vöst 1 , Markus Drees , Christian Jandl , Alexander Pöthig , Nicola P. M. Casati 2 , Wolfgang Scherer 1 , Bernhard Rieger
Affiliation
|
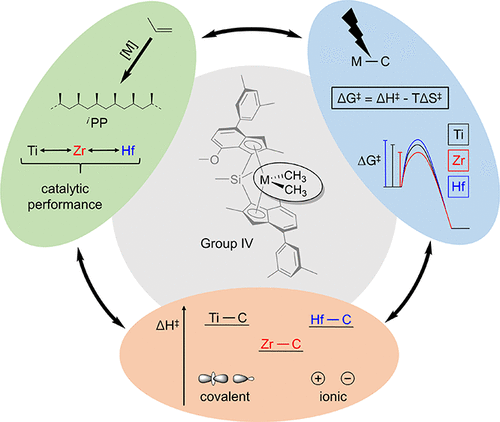
This contribution provides the first detailed analysis of the nature of the M–C σ-bond of three alkylated, isostructural group 4 (M = Ti, Zr, Hf) metallocenes, thereby elucidating individual peculiarities of each metal center in the catalytic conversion of olefins. Therefore, the subtle electronic differences of the individual M–C σ-bonds, which are considered crucial for several subprocesses in the coordinative polymerization of olefins, were examined by detailed experimental charge density studies. These studies provided measures of the increasing ionic character of the M–C bonds along the group 4 elements (Ti–C < Zr–C < Hf–C). These results are further supported by high-pressure diffraction studies showing that the predominantly ionic Hf–C bond is more compressible than the more covalent Zr–C bond in line with a smaller degree of electron localization in the valence shell of the hafnium relative to the zirconium atom along the M–C bond directions. The Ti–C bond displays the largest degree of electron localization in these group 4 metallocenes as witnessed by a pronounced bonded charge concentration in the valence shell of the titanium atom–a rare phenomenon in transition metal alkyls. All findings were then complemented by experimental and theoretical studies of the kinetic aspects of M–C σ-bond cleavage in group 4 metallocenes. These studies show that the entropy of activation is distinctly more negative for a Zr–C relative to a Hf–C bond dissociation. The combined results of the kinetic and electronic analysis herein shed new light on the different catalytic behavior of group 4 metallocenes with regard to the applied transition metal atom. In this context, deviations between zirconium- and hafnium-based catalysts concerning the catalytic activity and the stereoregularities became clearly explainable, just as the well-known “hafnium-effect” in the production of extraordinarily high molecular weight polypropylenes.
中文翻译:

第4组茂金属催化的幕后故事:金属-碳键的检查
该贡献首次详细分析了三个烷基化的同构第4族金属茂(M = Ti,Zr,Hf)茂金属的MC键的性质,从而阐明了烯烃催化转化中每个金属中心的独特性。 。因此,通过详细的实验电荷密度研究,研究了单个M–Cσ键的细微电子差异,这些差异被认为对烯烃配位聚合中的多个子过程至关重要。这些研究提供了沿着第4组元素(Ti-C <Zr-C <Hf-C)增加的M-C键离子特性的措施。这些结果得到高压衍射研究的进一步支持,显示出以离子为主的Hf–C键比具有更高共价键的Zr–C键具有更高的可压缩性,这是因为relative的化合价壳中的电子局部化程度相对于Hf–C键更小。沿M–C键方向的锆原子。Ti-C键在这些第4组金属茂中显示出最大程度的电子定位,这在钛原子的价态壳层中具有明显的键合电荷浓度即可证明,这是过渡金属烷基中罕见的现象。然后,对第4组茂金属中M–Cσ键断裂的动力学方面的实验和理论研究对所有发现进行了补充。这些研究表明,与Hf-C键解离相比,Zr-C的激活熵明显更负。本文动力学和电子分析的综合结果为第4组茂金属在所施加的过渡金属原子方面的不同催化行为提供了新的思路。在这种情况下,锆和ha基催化剂之间关于催化活性和立构规整度的偏差就可以清楚地解释,就像在生产超高分子量聚丙烯中众所周知的“ ha效应”一样。
更新日期:2018-08-16
中文翻译:

第4组茂金属催化的幕后故事:金属-碳键的检查
该贡献首次详细分析了三个烷基化的同构第4族金属茂(M = Ti,Zr,Hf)茂金属的MC键的性质,从而阐明了烯烃催化转化中每个金属中心的独特性。 。因此,通过详细的实验电荷密度研究,研究了单个M–Cσ键的细微电子差异,这些差异被认为对烯烃配位聚合中的多个子过程至关重要。这些研究提供了沿着第4组元素(Ti-C <Zr-C <Hf-C)增加的M-C键离子特性的措施。这些结果得到高压衍射研究的进一步支持,显示出以离子为主的Hf–C键比具有更高共价键的Zr–C键具有更高的可压缩性,这是因为relative的化合价壳中的电子局部化程度相对于Hf–C键更小。沿M–C键方向的锆原子。Ti-C键在这些第4组金属茂中显示出最大程度的电子定位,这在钛原子的价态壳层中具有明显的键合电荷浓度即可证明,这是过渡金属烷基中罕见的现象。然后,对第4组茂金属中M–Cσ键断裂的动力学方面的实验和理论研究对所有发现进行了补充。这些研究表明,与Hf-C键解离相比,Zr-C的激活熵明显更负。本文动力学和电子分析的综合结果为第4组茂金属在所施加的过渡金属原子方面的不同催化行为提供了新的思路。在这种情况下,锆和ha基催化剂之间关于催化活性和立构规整度的偏差就可以清楚地解释,就像在生产超高分子量聚丙烯中众所周知的“ ha效应”一样。















































 京公网安备 11010802027423号
京公网安备 11010802027423号