当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Analysis of Autoinduction, Inhibition, and Autoinhibition in a Rh-Catalyzed C–C Cleavage: Mechanism of Decyanative Aryl Silylation
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-08-14 00:00:00 , DOI: 10.1021/acscatal.8b02809 Eric C. Keske 1 , Thomas H. West 1 , Guy C. Lloyd-Jones 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-08-14 00:00:00 , DOI: 10.1021/acscatal.8b02809 Eric C. Keske 1 , Thomas H. West 1 , Guy C. Lloyd-Jones 1
Affiliation
|
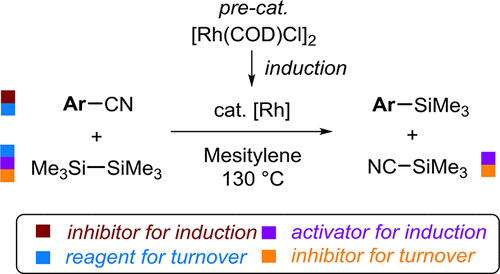
The mechanism the Chatani–Tobisu rhodium-catalyzed decyanative silylation of aryl nitriles by hexamethyldisilane (Me3Si-SiMe3) has been investigated by in situ NMR spectroscopy. The production of Ar-SiMe3 evolves in three distinct phases: slow catalyst induction is followed by a period of rapidly accelerating turnover and then, after approximately three catalyst turnovers, the onset of progressive inhibition. The processes giving rise to these phenomena have been elucidated by isotopic labeling (13C/15N) and kinetic analysis, and it is shown that, in addition to facilitating catalyst turnover to generate Ar-SiMe3, the reactants serve other roles. Me3Si-SiMe3 functions as a slow exogenous precatalyst activator and as a moderately powerful catalyst inhibitor. In contrast, ArCN acts as a precatalyst inhibitor. Moreover, the coproduct from the reaction (trimethylsilyl cyanide, Me3SiCN) acts as a powerful endogenous precatalyst activator and catalyst inhibitor, together giving rise to sigmoidal temporal concentration profiles for [Ar-SiMe3]. Kinetic studies of the reaction during the phase of progressive inhibition suggest that, for a given initial catalyst concentration, the maximum rate of turnover is achieved when the concentrations of [Me3Si-SiMe3] and [ArCN] partition the Rh equally between two major resting states, one on-cycle, the other off-cycle. The off-cycle resting-state was identified as a Rh(III) complex: (Me3Si)3Rh(CN-SiMe3)3, as confirmed by independent synthesis and isotopic labeling (13C/15N); the on-cycle resting state has been tentatively assigned as (Me3Si-NC)3RhAr. Overall, the results indicate that the catalytic process can, in principle, be made much more efficient by engendering a pathway or process for Me3SiCN sequestration.
中文翻译:

Rh催化的C–C裂解中的自诱导,抑制和自抑制分析:脱氰芳基甲硅烷基化的机理
已通过原位NMR光谱研究了Chatani–Tobisu铑催化的六甲基二硅烷(Me 3 Si-SiMe 3)芳基腈的脱氰甲硅烷基化反应。Ar-SiMe 3的产生分为三个不同的阶段:缓慢的催化剂诱导,随后是一个快速加速的周转期,然后,在大约三个催化剂周转后,开始进行逐步抑制。同位素标记(13 C / 15 N)和动力学分析已经阐明了引起这些现象的过程,结果表明,除了促进催化剂转化生成Ar-SiMe 3以外,反应物还起其他作用。我3 Si-SiMe3用作缓慢的外源性预催化剂活化剂和中等强度的催化剂抑制剂。相反,ArCN充当前催化剂抑制剂。此外,反应的副产物(三甲基甲硅烷基氰化物,Me 3 SiCN)充当强大的内源性预催化剂活化剂和催化剂抑制剂,共同产生了[Ar-SiMe 3 ]的S型时间浓度分布。在渐进抑制阶段反应的动力学研究表明,对于给定的初始催化剂浓度,当[Me 3 Si-SiMe 3]和[ArCN]在两个主要的静止状态(一个处于循环状态,另一个处于停止状态)之间平均分配Rh。通过独立的合成和同位素标记(13 C / 15 N)证实,循环外的静止状态被鉴定为Rh(III)络合物:(Me 3 Si)3 Rh(CN-SiMe 3)3。暂态上的静止状态已被暂定为(Me 3 Si-NC)3 RhAr。总体而言,结果表明,原则上可以通过产生Me 3 SiCN螯合的途径或方法使催化过程更有效。
更新日期:2018-08-14
中文翻译:

Rh催化的C–C裂解中的自诱导,抑制和自抑制分析:脱氰芳基甲硅烷基化的机理
已通过原位NMR光谱研究了Chatani–Tobisu铑催化的六甲基二硅烷(Me 3 Si-SiMe 3)芳基腈的脱氰甲硅烷基化反应。Ar-SiMe 3的产生分为三个不同的阶段:缓慢的催化剂诱导,随后是一个快速加速的周转期,然后,在大约三个催化剂周转后,开始进行逐步抑制。同位素标记(13 C / 15 N)和动力学分析已经阐明了引起这些现象的过程,结果表明,除了促进催化剂转化生成Ar-SiMe 3以外,反应物还起其他作用。我3 Si-SiMe3用作缓慢的外源性预催化剂活化剂和中等强度的催化剂抑制剂。相反,ArCN充当前催化剂抑制剂。此外,反应的副产物(三甲基甲硅烷基氰化物,Me 3 SiCN)充当强大的内源性预催化剂活化剂和催化剂抑制剂,共同产生了[Ar-SiMe 3 ]的S型时间浓度分布。在渐进抑制阶段反应的动力学研究表明,对于给定的初始催化剂浓度,当[Me 3 Si-SiMe 3]和[ArCN]在两个主要的静止状态(一个处于循环状态,另一个处于停止状态)之间平均分配Rh。通过独立的合成和同位素标记(13 C / 15 N)证实,循环外的静止状态被鉴定为Rh(III)络合物:(Me 3 Si)3 Rh(CN-SiMe 3)3。暂态上的静止状态已被暂定为(Me 3 Si-NC)3 RhAr。总体而言,结果表明,原则上可以通过产生Me 3 SiCN螯合的途径或方法使催化过程更有效。































 京公网安备 11010802027423号
京公网安备 11010802027423号