当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
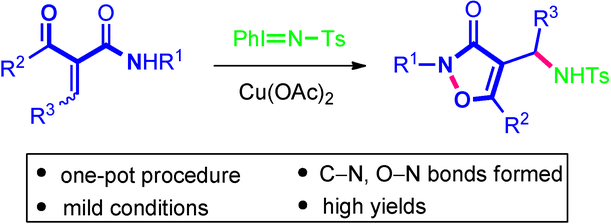
Copper‐Nitrene Triggered Annulation of α‐Acyl Cinnamides: Access to Isoxazol‐3(2H)‐ones
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2016-01-22 , DOI: 10.1002/adsc.201500990 Mangfei Yu , Qian Zhang , Gong Li , Jingwen Yuan , Ning Zhang , Rui Zhang , Yongjiu Liang , Dewen Dong
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2016-01-22 , DOI: 10.1002/adsc.201500990 Mangfei Yu , Qian Zhang , Gong Li , Jingwen Yuan , Ning Zhang , Rui Zhang , Yongjiu Liang , Dewen Dong
|
A facile and efficient one‐pot synthesis of isoxazol‐3(2H)‐ones has been developed starting from α‐acyl cinnamides and tosyliminophenyliodinane catalyzed by copper(II) acetate [Cu(OAc)2] under very mild conditions involving a tandem aza‐Michael addition and intramolecular cyclization sequence.
中文翻译:

铜-硝烯引发的α-酰基肉桂酸酯环化反应:使用异恶唑3(2H)-酮
从α-酰基肉桂酸酯和甲苯磺酸苯基碘丁烷在乙酸铜[II] [Cu(OAc)2 ]催化下,在非常温和的条件下(串联)合成,已开发了一种简便高效的异恶唑-3(2 H)-酮一锅合成方法氮杂-迈克尔加成和分子内环化序列。
更新日期:2016-01-22
中文翻译:

铜-硝烯引发的α-酰基肉桂酸酯环化反应:使用异恶唑3(2H)-酮
从α-酰基肉桂酸酯和甲苯磺酸苯基碘丁烷在乙酸铜[II] [Cu(OAc)2 ]催化下,在非常温和的条件下(串联)合成,已开发了一种简便高效的异恶唑-3(2 H)-酮一锅合成方法氮杂-迈克尔加成和分子内环化序列。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号