当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Coordination-Driven Self-Assembled ZnII6-LnIII3 Metallocycles Based on a Salicylamide Imine Ligand: Synthesis, Structure, and Selective Luminescence Enhancement Induced by OAc–
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-08-13 00:00:00 , DOI: 10.1021/acs.inorgchem.8b01525 Xue-Qin Song 1 , Cai-Yun Wang 1 , Huan-Huan Meng 1 , Ali Alhafeez Abdulrheem Shamshoom 1 , Wei-sheng Liu 2
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-08-13 00:00:00 , DOI: 10.1021/acs.inorgchem.8b01525 Xue-Qin Song 1 , Cai-Yun Wang 1 , Huan-Huan Meng 1 , Ali Alhafeez Abdulrheem Shamshoom 1 , Wei-sheng Liu 2
Affiliation
|
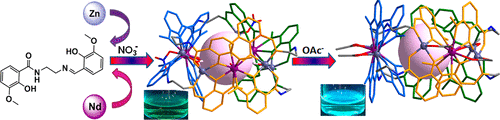
Five heterometallic ZnII6-LnIII3 macrocycles based on a salicylamide imine multidentate unsymmetrical ligand H2L [1-(2-hydroxy-3-methoxy-benzamido)-2-(2-hydroxy-3-methoxy-benzylideneamino)-ethane] have been prepared via a coordination-driven self-assembly strategy. Single-crystal X-ray diffraction analysis reveals that the five metallocycles are isomorphic with a formula of [Zn6Ln3L6(OH)2(NO3)4(H2O)]·3NO3·nCH3CN (ZnLn-1, where Ln = Pr, Nd, Eu, Tb, or Yb; for ZnPr-1, n = 4; for ZnNd-1, ZnEu-1, and ZnTb-1, n = 2; for ZnYb-1, n = 3), where six octadentate ligands L2– and two in situ-formed μ2-OH– ions bridged the alternating ZnII-LnIII-ZnII subunits into a macrocycle. Along with the structural novelty, ZnNd-1 shows distinctive enhanced emission in the visible and near-infrared range upon addition of OAc–. On the basis of ultraviolet–visible spectroscopy, 1H nuclear magnetic resonance, single-crystal X-ray diffraction, and high-resolution electrospray ionization mass spectrometry, we deduced that this emission enhancement could be attributed to the synergistic effect of TICT and the absent nonradiative transition of μ2-OH– induced distinctively by OAc– bridging. Our results demonstrate that the NdIII-containing heterometallic macrocycle can act as a host for anion exchanging and provide a nice example of heterometallic macrocycles with interesting properties and potential applications.
中文翻译:

基于水杨酰胺亚胺配体的配位驱动自组装Zn II 6 -Ln III 3金属环:OAc诱导的合成,结构和选择性发光增强–
五个杂金属锌II 6 -Ln III 3个大环基于水杨酰胺亚胺多齿配位体的非对称ħ 2 L [1-(2-羟基-3-甲氧基-苯甲酰氨基)-2-(2-羟基-3-甲氧基-苯亚甲基氨基) -乙烷]是通过协调驱动的自组装策略制备的。X射线单晶衍射分析表明,这五个金属环是同构的,分子式为[Zn 6 Ln 3 L 6(OH)2(NO 3)4(H 2 O)]·3NO 3 · n CH 3 CN(ZnLn-1,其中Ln = Pr,Nd,Eu,Tb或Yb;对于ZnPr-1,n= 4;对于ZnPr-1,n= 4。对于ZnNd-1,ZnEu-1和ZnTb-1,n = 2;对于ZnNd-1,ZnEu-1和ZnTb-1,n = 2。对于ZnYb-1 ,Ñ = 3),其中6个octadentate配体L 2-中和两个原位形成的μ 2 -OH -离子桥的交流锌II -Ln III -Zn II亚基插入一个大环。随着结构的新颖性,添加OAc时,ZnNd-1在可见光和近红外范围内显示出独特的增强发射–。在紫外可见光谱,1 H核磁共振,单晶X射线衍射和高分辨率电喷雾电离质谱的基础上,我们推论出这种发射增强可归因于TICT的协同作用和缺乏μ的无辐射跃迁2 -OH -通过OAC区别诱导-桥接。我们的结果表明,含Nd III的杂金属大环化合物可以充当阴离子交换的主体,并提供具有有趣的性质和潜在应用的杂金属大环化合物的一个很好的例子。
更新日期:2018-08-13
中文翻译:

基于水杨酰胺亚胺配体的配位驱动自组装Zn II 6 -Ln III 3金属环:OAc诱导的合成,结构和选择性发光增强–
五个杂金属锌II 6 -Ln III 3个大环基于水杨酰胺亚胺多齿配位体的非对称ħ 2 L [1-(2-羟基-3-甲氧基-苯甲酰氨基)-2-(2-羟基-3-甲氧基-苯亚甲基氨基) -乙烷]是通过协调驱动的自组装策略制备的。X射线单晶衍射分析表明,这五个金属环是同构的,分子式为[Zn 6 Ln 3 L 6(OH)2(NO 3)4(H 2 O)]·3NO 3 · n CH 3 CN(ZnLn-1,其中Ln = Pr,Nd,Eu,Tb或Yb;对于ZnPr-1,n= 4;对于ZnPr-1,n= 4。对于ZnNd-1,ZnEu-1和ZnTb-1,n = 2;对于ZnNd-1,ZnEu-1和ZnTb-1,n = 2。对于ZnYb-1 ,Ñ = 3),其中6个octadentate配体L 2-中和两个原位形成的μ 2 -OH -离子桥的交流锌II -Ln III -Zn II亚基插入一个大环。随着结构的新颖性,添加OAc时,ZnNd-1在可见光和近红外范围内显示出独特的增强发射–。在紫外可见光谱,1 H核磁共振,单晶X射线衍射和高分辨率电喷雾电离质谱的基础上,我们推论出这种发射增强可归因于TICT的协同作用和缺乏μ的无辐射跃迁2 -OH -通过OAC区别诱导-桥接。我们的结果表明,含Nd III的杂金属大环化合物可以充当阴离子交换的主体,并提供具有有趣的性质和潜在应用的杂金属大环化合物的一个很好的例子。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号