当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lithium Nitrate Solvation Chemistry in Carbonate Electrolyte Sustains High‐Voltage Lithium Metal Batteries
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-09-07 , DOI: 10.1002/anie.201807034 Chong Yan 1 , Yu-Xing Yao 2 , Xiang Chen 2 , Xin-Bing Cheng 2 , Xue-Qiang Zhang 2 , Jia-Qi Huang 1 , Qiang Zhang 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-09-07 , DOI: 10.1002/anie.201807034 Chong Yan 1 , Yu-Xing Yao 2 , Xiang Chen 2 , Xin-Bing Cheng 2 , Xue-Qiang Zhang 2 , Jia-Qi Huang 1 , Qiang Zhang 2
Affiliation
|
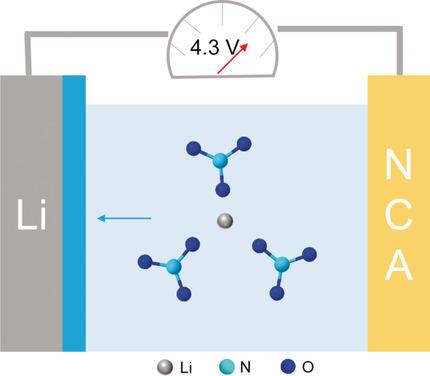
The lithium metal anode is regarded as a promising candidate in next‐generation energy storage devices. Lithium nitrate (LiNO3) is widely applied as an effective additive in ether electrolyte to increase the interfacial stability in batteries containing lithium metal anodes. However, because of its poor solubility LiNO3 is rarely utilized in the high‐voltage window provided by carbonate electrolyte. Dissolution of LiNO3 in carbonate electrolyte is realized through an effective solvation regulation strategy. LiNO3 can be directly dissolved in an ethylene carbonate/diethyl carbonate electrolyte mixture by adding trace amounts of copper fluoride as a dissolution promoter. LiNO3 protects the Li metal anode in a working high‐voltage Li metal battery. When a LiNi0.80Co0.15Al0.05O2 cathode is paired with a Li metal anode, an extraordinary capacity retention of 53 % is achieved after 300 cycles (13 % after 200 cycles for LiNO3‐free electrolyte) and a very high average Coulombic efficiency above 99.5 % is achieved at 0.5 C. The solvation chemistry of LiNO3‐containing carbonate electrolyte may sustain high‐voltage Li metal anodes operating in corrosive carbonate electrolytes.
中文翻译:

碳酸盐电解质中的硝酸锂溶剂化化学支持高压锂金属电池
锂金属阳极被认为是下一代储能设备中有希望的候选者。硝酸锂(LiNO 3)被广泛用作醚电解质中的有效添加剂,以提高含锂金属阳极的电池的界面稳定性。然而,由于其溶解度差,LiNO 3很少用于碳酸盐电解质提供的高压窗口中。LiNO 3在碳酸盐电解质中的溶解是通过有效的溶剂化调节策略实现的。通过添加痕量的氟化铜作为溶解促进剂,可以将LiNO 3直接溶解在碳酸亚乙酯/碳酸二乙酯的电解质混合物中。锂3保护正在使用的高压锂金属电池中的锂金属阳极。当将LiNi 0.80 Co 0.15 Al 0.05 O 2阴极与Li金属阳极配对时,在300次循环后,容量保持率达到了53%(对于不含LiNO 3的电解质,经过200次循环后,达到了13%),并且平均库仑电量非常高在0.5 C时,效率可达到99.5%以上。含LiNO 3的碳酸盐电解质的溶剂化化学反应可维持在腐蚀性碳酸盐电解质中运行的高压锂金属阳极。
更新日期:2018-09-07
中文翻译:

碳酸盐电解质中的硝酸锂溶剂化化学支持高压锂金属电池
锂金属阳极被认为是下一代储能设备中有希望的候选者。硝酸锂(LiNO 3)被广泛用作醚电解质中的有效添加剂,以提高含锂金属阳极的电池的界面稳定性。然而,由于其溶解度差,LiNO 3很少用于碳酸盐电解质提供的高压窗口中。LiNO 3在碳酸盐电解质中的溶解是通过有效的溶剂化调节策略实现的。通过添加痕量的氟化铜作为溶解促进剂,可以将LiNO 3直接溶解在碳酸亚乙酯/碳酸二乙酯的电解质混合物中。锂3保护正在使用的高压锂金属电池中的锂金属阳极。当将LiNi 0.80 Co 0.15 Al 0.05 O 2阴极与Li金属阳极配对时,在300次循环后,容量保持率达到了53%(对于不含LiNO 3的电解质,经过200次循环后,达到了13%),并且平均库仑电量非常高在0.5 C时,效率可达到99.5%以上。含LiNO 3的碳酸盐电解质的溶剂化化学反应可维持在腐蚀性碳酸盐电解质中运行的高压锂金属阳极。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号