当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Accessing Polysubstituted Quinazolines via Nickel Catalyzed Acceptorless Dehydrogenative Coupling
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-08-09 00:00:00 , DOI: 10.1021/acs.joc.8b01479
Seuli Parua 1 , Rina Sikari 1 , Suman Sinha 1 , Gargi Chakraborty 1 , Rakesh Mondal 1 , Nanda D. Paul 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-08-09 00:00:00 , DOI: 10.1021/acs.joc.8b01479
Seuli Parua 1 , Rina Sikari 1 , Suman Sinha 1 , Gargi Chakraborty 1 , Rakesh Mondal 1 , Nanda D. Paul 1
Affiliation
|
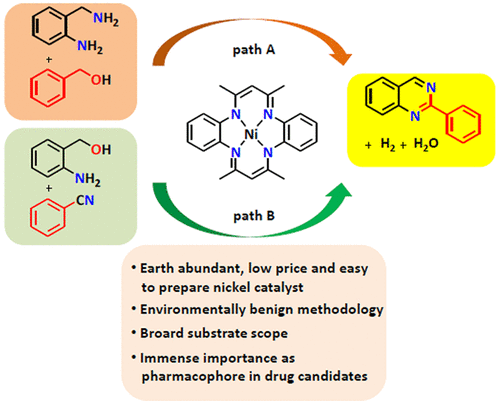
Two environmentally benign methods for the synthesis of quinazolines via acceptorless dehydrogenative coupling of 2-aminobenzylamine with benzyl alcohol (Path A) and 2-aminobenzylalcohol with benzonitrile (Path B), catalyzed by cheap, earth abundant and easy to prepare nickel catalysts, containing tetraaza macrocyclic ligands (tetramethyltetraaza[14]annulene (MeTAA) or 6,15-dimethyl-8,17-diphenyltetraaza[14]annulene (MePhTAA)) are reported. A wide variety of substituted quinazolines were synthesized in moderate to high yields starting from cheap and easily available starting precursors. A few control reactions were performed to understand the mechanism and to establish the acceptorless dehydrogenative nature of the catalytic reactions.
中文翻译:

通过镍催化的无受体脱氢偶联作用获得多取代的喹唑啉
通过2-氨基苄胺与苄醇的无受体脱氢偶联(路径A)和2-氨基苄基醇与苄腈的无受体脱氢偶联(路径B)来合成喹唑啉的两种对环境有益的方法,它们是由廉价的,富含地球的,易于制备的含四氮杂的镍催化剂催化的报道了大环配体(四甲基四氮杂[14]环烯(MeTAA)或6,15-二甲基-8,17-二苯基四氮杂[14]环烯(MePhTAA))。从廉价且容易获得的起始前体开始,以中等至高收率合成了多种取代的喹唑啉。进行了一些控制反应,以了解机理并建立催化反应的无受体脱氢性质。
更新日期:2018-08-09
中文翻译:

通过镍催化的无受体脱氢偶联作用获得多取代的喹唑啉
通过2-氨基苄胺与苄醇的无受体脱氢偶联(路径A)和2-氨基苄基醇与苄腈的无受体脱氢偶联(路径B)来合成喹唑啉的两种对环境有益的方法,它们是由廉价的,富含地球的,易于制备的含四氮杂的镍催化剂催化的报道了大环配体(四甲基四氮杂[14]环烯(MeTAA)或6,15-二甲基-8,17-二苯基四氮杂[14]环烯(MePhTAA))。从廉价且容易获得的起始前体开始,以中等至高收率合成了多种取代的喹唑啉。进行了一些控制反应,以了解机理并建立催化反应的无受体脱氢性质。































 京公网安备 11010802027423号
京公网安备 11010802027423号