Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Biotransformation, Using Recombinant CYP450-Expressing Baker’s Yeast Cells, Identifies a Novel CYP2D6.10A122V Variant Which Is a Superior Metabolizer of Codeine to Morphine Than the Wild-Type Enzyme
ACS Omega ( IF 3.7 ) Pub Date : 2018-08-09 00:00:00 , DOI: 10.1021/acsomega.8b00809 Ibidapo S. Williams 1 , Linda Gatchie 1 , Sandip B. Bharate 2 , Bhabatosh Chaudhuri 1
ACS Omega ( IF 3.7 ) Pub Date : 2018-08-09 00:00:00 , DOI: 10.1021/acsomega.8b00809 Ibidapo S. Williams 1 , Linda Gatchie 1 , Sandip B. Bharate 2 , Bhabatosh Chaudhuri 1
Affiliation
|
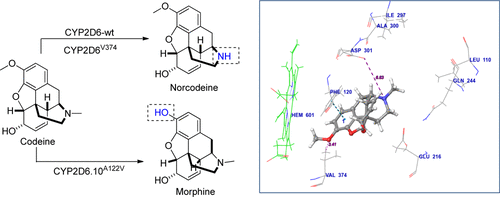
CYP2D6, a cytochrome P450 (CYP) enzyme, metabolizes codeine to morphine. Within the human body, 0–15% of codeine undergoes O-demethylation by CYP2D6 to form morphine, a far stronger analgesic than codeine. Genetic polymorphisms in wild-type CYP2D6 (CYP2D6-wt) are known to cause poor-to-extensive metabolism of codeine and other CYP2D6 substrates. We have established a platform technology that allows stable expression of human CYP genes from chromosomal loci of baker’s yeast cells. Four CYP2D6 alleles, (i) chemically synthesized CYP2D6.1, (ii) chemically synthesized CYP2D6-wt, (iii) chemically synthesized CYP2D6.10, and (iv) a novel CYP2D6.10 variant CYP2D6-C (i.e., CYP2D6.10A122V) isolated from a liver cDNA library, were cloned for chromosomal integration in yeast cells. When expressed in yeast, CYP2D6.10 enzyme shows weak activity compared with CYP2D6-wt and CYP2D6.1 which have moderate activity, as reported earlier. Surprisingly, however, the CYP2D6-C enzyme is far more active than CYP2D6.10. More surprisingly, although CYP2D6.10 is a known low metabolizer of codeine, yeast cells expressing CYP2D6-C transform >70% of codeine to morphine, which is more than twice that of cells expressing the extensive metabolizers, CYP2D6.1, and CYP2D6-wt. The latter two enzymes predominantly catalyze formation of codeine’s N-demethylation product, norcodeine, with >55% yield. Molecular modeling studies explain the specificity of CYP2D6-C for O-demethylation, validating observed experimental results. The yeast-based CYP2D6 expression systems, described here, could find generic use in CYP2D6-mediated drug metabolism and also in high-yield chemical reactions that allow the formation of regio-specific dealkylation products.
中文翻译:

使用重组表达CYP450的酵母细胞进行生物转化,鉴定了一种新型CYP2D6.10 A122V变体,该变体是可待因比吗啡比野生型酶更强的代谢者
CYP2D6是一种细胞色素P450(CYP)酶,将可待因代谢为吗啡。在人体中,0-15%的可待因被CYP2D6进行O-去甲基化反应,形成吗啡,比可待因强得多。已知野生型CYP2D6(CYP2D6-wt)中的遗传多态性导致可待因和其他CYP2D6底物的代谢范围不广。我们已经建立了一种平台技术,该技术可以从面包酵母细胞的染色体基因座稳定表达人CYP基因。四个CYP2D6等位基因,(i)化学合成的CYP2D6.1,(ii)化学合成的CYP2D6-wt,(iii)化学合成的CYP2D6.10,和(iv)新的CYP2D6.10 CYP2D6-C变体(即CYP2D6.10 A122V从肝脏cDNA文库中分离出来的cDNA克隆到酵母细胞中进行染色体整合。如先前报道,当在酵母中表达时,CYP2D6.10酶的活性比具有中等活性的CYP2D6-wt和CYP2D6.1弱。但是,令人惊讶的是,CYP2D6-C酶的活性远高于CYP2D6.10。更令人惊讶的是,尽管CYP2D6.10是可待因的一种低代谢物,表达CYP2D6-C的酵母细胞将> 70%的可待因转化为吗啡,是表达广泛代谢物CYP2D6.1和CYP2D6-的细胞的两倍以上。重量 后两种酶主要催化可待因的N-去甲基化产物降钙素的形成,收率> 55%。分子模型研究解释了CYP2D6-C对O-去甲基化的特异性,验证了观察到的实验结果。
更新日期:2018-08-09
中文翻译:

使用重组表达CYP450的酵母细胞进行生物转化,鉴定了一种新型CYP2D6.10 A122V变体,该变体是可待因比吗啡比野生型酶更强的代谢者
CYP2D6是一种细胞色素P450(CYP)酶,将可待因代谢为吗啡。在人体中,0-15%的可待因被CYP2D6进行O-去甲基化反应,形成吗啡,比可待因强得多。已知野生型CYP2D6(CYP2D6-wt)中的遗传多态性导致可待因和其他CYP2D6底物的代谢范围不广。我们已经建立了一种平台技术,该技术可以从面包酵母细胞的染色体基因座稳定表达人CYP基因。四个CYP2D6等位基因,(i)化学合成的CYP2D6.1,(ii)化学合成的CYP2D6-wt,(iii)化学合成的CYP2D6.10,和(iv)新的CYP2D6.10 CYP2D6-C变体(即CYP2D6.10 A122V从肝脏cDNA文库中分离出来的cDNA克隆到酵母细胞中进行染色体整合。如先前报道,当在酵母中表达时,CYP2D6.10酶的活性比具有中等活性的CYP2D6-wt和CYP2D6.1弱。但是,令人惊讶的是,CYP2D6-C酶的活性远高于CYP2D6.10。更令人惊讶的是,尽管CYP2D6.10是可待因的一种低代谢物,表达CYP2D6-C的酵母细胞将> 70%的可待因转化为吗啡,是表达广泛代谢物CYP2D6.1和CYP2D6-的细胞的两倍以上。重量 后两种酶主要催化可待因的N-去甲基化产物降钙素的形成,收率> 55%。分子模型研究解释了CYP2D6-C对O-去甲基化的特异性,验证了观察到的实验结果。





































 京公网安备 11010802027423号
京公网安备 11010802027423号