当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conversion of N-Acetylglucosamine to Protected Amino Acid over Ru/C Catalyst
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2018-08-09 00:00:00 , DOI: 10.1021/acssuschemeng.8b02951 Kota Techikawara , Hirokazu Kobayashi , Atsushi Fukuoka
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2018-08-09 00:00:00 , DOI: 10.1021/acssuschemeng.8b02951 Kota Techikawara , Hirokazu Kobayashi , Atsushi Fukuoka
|
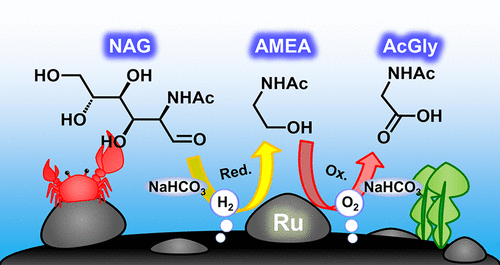
Chitin is the most abundant marine biomass, containing nitrogen atoms in its monomer units, N-acetylglucosamine (NAG). Thus, NAG is a potential feedstock for the production of renewable organonitrogen chemicals. Here, we report a synthetic pathway of a widely used standard amino acid in a protected form, acetylglycine (AcGly), from NAG via two steps: conversion of NAG to N-acetylmonoethanolamine (AMEA) and its transformation to AcGly. In the first step, a Ru/C catalyst converts NAG to AMEA in an NaHCO3 aqueous solution under H2 pressure through a retro-aldol reaction and hydrogenation reaction. Model reactions and density functional theory calculations for this reaction have revealed that resonance stabilization of the acetamido group of NAG avoids its isomerization proceeding to side reactions, which is different from glucose regardless of their similar structures. For the second step of the synthesis of AcGly from AMEA under O2 pressure, the same combination of Ru/C and NaHCO3 is applicable. A mechanistic study has shown that the oxidation reaction is not due to autoxidation but by catalysis of Ru/C. Characterization of the Ru catalyst has revealed that the Ru species is present as small hydrous RuO2 clusters with hydroxo, aqua, μ-hydroxo, and μ-oxo ligands in the presence of O2. This species may work as a catalyst for the oxidation of AMEA.
中文翻译:

Ru / C催化剂将N-乙酰氨基葡萄糖转化为受保护的氨基酸
几丁质是最丰富的海洋生物质,在其单体单元中含有氮原子N-乙酰氨基葡萄糖(NAG)。因此,NAG是生产可再生有机氮化学品的潜在原料。在这里,我们通过两个步骤报告了来自NAG的一种广泛使用的标准氨基酸的保护途径,即乙酰甘氨酸(AcGly)的合成途径:将NAG转化为N-乙酰基单乙醇胺(AMEA)并将其转化为AcGly。第一步,Ru / C催化剂在H 2下于NaHCO 3水溶液中将NAG转化为AMEA通过逆醛醇缩合反应和加氢反应产生压力。模型反应和该反应的密度泛函理论计算表明,NAG的乙酰氨基基团的共振稳定化可避免其异构化发展为副反应,无论其结构如何,该副反应均与葡萄糖不同。对于在O 2压力下由AMEA合成AcGly的第二步,可以使用Ru / C和NaHCO 3的相同组合。机理研究表明,氧化反应不是由于自氧化作用,而是由于Ru / C的催化作用。Ru催化剂的表征表明,Ru物种以O的形式以带有羟基,水基,μ-羟基和μ-氧代配体的小含水RuO 2簇形式存在。2。该物质可以用作AMEA氧化的催化剂。
更新日期:2018-08-09
中文翻译:

Ru / C催化剂将N-乙酰氨基葡萄糖转化为受保护的氨基酸
几丁质是最丰富的海洋生物质,在其单体单元中含有氮原子N-乙酰氨基葡萄糖(NAG)。因此,NAG是生产可再生有机氮化学品的潜在原料。在这里,我们通过两个步骤报告了来自NAG的一种广泛使用的标准氨基酸的保护途径,即乙酰甘氨酸(AcGly)的合成途径:将NAG转化为N-乙酰基单乙醇胺(AMEA)并将其转化为AcGly。第一步,Ru / C催化剂在H 2下于NaHCO 3水溶液中将NAG转化为AMEA通过逆醛醇缩合反应和加氢反应产生压力。模型反应和该反应的密度泛函理论计算表明,NAG的乙酰氨基基团的共振稳定化可避免其异构化发展为副反应,无论其结构如何,该副反应均与葡萄糖不同。对于在O 2压力下由AMEA合成AcGly的第二步,可以使用Ru / C和NaHCO 3的相同组合。机理研究表明,氧化反应不是由于自氧化作用,而是由于Ru / C的催化作用。Ru催化剂的表征表明,Ru物种以O的形式以带有羟基,水基,μ-羟基和μ-氧代配体的小含水RuO 2簇形式存在。2。该物质可以用作AMEA氧化的催化剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号