当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
First Principles Investigation of HCl, H2, and Chlorosilane Adsorption on Cu3Si Surfaces with Applications for Polysilicon Production
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-08-23 , DOI: 10.1021/acs.jpcc.8b04460 Shwetank Yadav , Chandra Veer Singh
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-08-23 , DOI: 10.1021/acs.jpcc.8b04460 Shwetank Yadav , Chandra Veer Singh
|
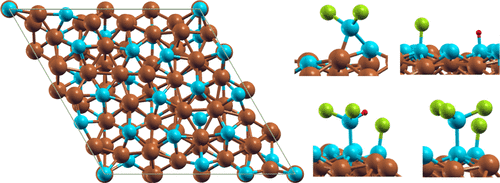
The hydrochlorination reaction used in polysilicon production is often catalyzed through copper-based materials which have been found to form the Cu3Si intermetallic under reaction conditions. The structure and composition of the η″ phase, present in the reaction temperature range, has been only recently derived. We conduct the first study of Cu3Si surfaces and their interaction with molecules. Two surfaces of the η″ phase were examined through density functional theory, a silicon-rich Si termination in hexagonal geometry and an equal-parts copper and silicon CuSi termination in honeycomb geometry. The adsorption of H2, HCl, SiCl2, dichlorosilane (SiH2Cl2 or DCS), trichlorosilane (SiHCl3 or TCS), and silicon tetrachloride (SiCl4 or STC) was tested on multiple sites. The SiCu termination favorably adsorbed only the SiCl2 molecule, where a bridge position between a Cu and Si atom produced the strongest adsorption. The Si-terminated surface dissociatively adsorbed HCl at all sites with almost equal adsorption energies. The SiCl2 adsorbed at all sites but greatly preferred a Cu–Si bridge site, where it produced the strongest adsorption of this study. The H2 molecule moved away from the surface for all sites. The STC molecule was unfavorable at sites with closely spaced Cu and Si atoms but dissociatively adsorbed at the other sites with more isolated Si atoms. The TCS molecule underwent dissociative chemisorption on all sites while the DCS molecule moved away from the surface for all site types. Therefore, the Si-terminated surface is preferred for molecular adsorption and appears to provide promising starting sites for surface hydrochlorination reactions.
中文翻译:

HCl,H 2和氯硅烷在Cu 3 Si表面吸附的第一原理研究以及在多晶硅生产中的应用
多晶硅生产中使用的氢氯化反应通常通过铜基材料催化,该铜基材料在反应条件下已形成Cu 3 Si金属间化合物。存在于反应温度范围内的η''相的结构和组成直到最近才被推导。我们对Cu 3 Si表面及其与分子的相互作用进行了首次研究。通过密度泛函理论检查了η''相的两个表面,即六角形几何形状的富硅Si端接和蜂窝状几何结构的等分铜和硅CuSi端接。H 2,HCl,SiCl 2,二氯硅烷(SiH 2 Cl 2)的吸附或DCS),三氯硅烷(SiHCl 3或TCS)和四氯化硅(SiCl 4或STC)在多个位置进行了测试。SiCu末端仅对SiCl 2分子有利地吸附,其中Cu和Si原子之间的桥键位置产生最强的吸附。硅末端的表面在所有位点都以几乎相等的吸附能解离地吸附HCl。SiCl 2吸附在所有位点上,但非常优选Cu-Si桥位点,在该位点该吸附作用最强。H 2分子从所有位置移离表面。STC分子在Cu和Si原子间距较近的位置处不利,但在其他Si原子较分离的位置处解离吸附。TCS分子在所有位点均经历解离化学吸附,而DCS分子对于所有位点类型均远离表面。因此,硅末端表面对于分子吸附是优选的,并且似乎为表面氢氯化反应提供了有希望的起始位点。
更新日期:2018-08-23
中文翻译:

HCl,H 2和氯硅烷在Cu 3 Si表面吸附的第一原理研究以及在多晶硅生产中的应用
多晶硅生产中使用的氢氯化反应通常通过铜基材料催化,该铜基材料在反应条件下已形成Cu 3 Si金属间化合物。存在于反应温度范围内的η''相的结构和组成直到最近才被推导。我们对Cu 3 Si表面及其与分子的相互作用进行了首次研究。通过密度泛函理论检查了η''相的两个表面,即六角形几何形状的富硅Si端接和蜂窝状几何结构的等分铜和硅CuSi端接。H 2,HCl,SiCl 2,二氯硅烷(SiH 2 Cl 2)的吸附或DCS),三氯硅烷(SiHCl 3或TCS)和四氯化硅(SiCl 4或STC)在多个位置进行了测试。SiCu末端仅对SiCl 2分子有利地吸附,其中Cu和Si原子之间的桥键位置产生最强的吸附。硅末端的表面在所有位点都以几乎相等的吸附能解离地吸附HCl。SiCl 2吸附在所有位点上,但非常优选Cu-Si桥位点,在该位点该吸附作用最强。H 2分子从所有位置移离表面。STC分子在Cu和Si原子间距较近的位置处不利,但在其他Si原子较分离的位置处解离吸附。TCS分子在所有位点均经历解离化学吸附,而DCS分子对于所有位点类型均远离表面。因此,硅末端表面对于分子吸附是优选的,并且似乎为表面氢氯化反应提供了有希望的起始位点。































 京公网安备 11010802027423号
京公网安备 11010802027423号