当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dioxygen Activation on Cu-MOR Zeolite: Theoretical Insights into the Formation of Cu2O and Cu3O3 Active Species
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-08-09 00:00:00 , DOI: 10.1021/acs.inorgchem.8b01329
M. Haris Mahyuddin 1 , Takahiro Tanaka , Aleksandar Staykov , Yoshihito Shiota , Kazunari Yoshizawa
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-08-09 00:00:00 , DOI: 10.1021/acs.inorgchem.8b01329
M. Haris Mahyuddin 1 , Takahiro Tanaka , Aleksandar Staykov , Yoshihito Shiota , Kazunari Yoshizawa
Affiliation
|
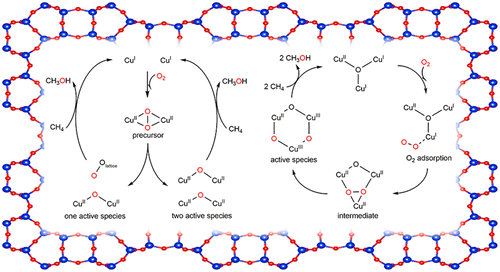
The utilization of low-cost and abundant oxygen (O2) as an oxidant in the activation of copper-exchanged zeolites is highly important for the direct, selective oxidation of methane to methanol at low temperatures. While two motifs of active sites, i.e., the [Cu2(μ-O)]2+ and [Cu3(μ-O)3]2+, have been experimentally observed in mordenite (MOR) zeolite, the mechanisms of their formation from the reaction of Cu-MOR with O2 are still unclear. In this study, we performed density functional theory (DFT) calculations for O2 activation over 2[Cu2]2+-MOR and [Cu3O]2+-MOR zeolites. For the reaction on the dicopper species, we found two possible reaction routes: O–O bond cleavage leading to (1) formation of a [Cu2(μ-O)]2+ active species and a trans-μ-1,2-peroxo-Si2 species and (2) simultaneous formation of two [Cu2(μ-O)]2+ active species neighboring to each other. These routes are both exothermic but require completely different O–O bond activation energies. For the reaction on the tricopper species, we suggest a peroxo-Cu3O species as the intermediate structure with two transition states (TSs) involved in the reaction. The first TS where a significant rearrangement of the tricopper site occurs is found to be rate-determining, while the second TS where the peroxo bond is cleaved results in a smaller activation barrier. This reaction, in contrast to the dicopper case, is slightly endothermic. The present study provides theoretical insights that may help design of better Cu-exchanged zeolite catalysts for methane hydroxylation to methanol.
中文翻译:

Cu-MOR沸石上的双氧活化:Cu 2 O和Cu 3 O 3活性物种形成的理论见解
在铜交换的沸石的活化中利用低成本和丰富的氧气(O 2)作为氧化剂对于在低温下将甲烷直接,选择性地氧化为甲醇非常重要。尽管在丝光沸石(MOR)中已通过实验观察到了活性位点的两个基序,即[Cu 2(μ-O)] 2+和[Cu 3(μ-O)3 ] 2+,但它们的机理Cu-MOR与O 2的反应形成的机理尚不清楚。在这项研究中,我们对2 [Cu 2 ] 2+ -MOR和[Cu 3 O]上的O 2活化进行了密度泛函理论(DFT)计算。2+ -MOR沸石。对于双铜物种的反应,我们发现了两种可能的反应途径:O-O键断裂导致(1)形成[Cu 2(μ-O)] 2+活性物种和反式-μ-1,2 -过氧-Si 2物种和(2)同时形成彼此相邻的两个[Cu 2(μ-O)] 2+活性物种。这些途径都是放热的,但需要完全不同的O–O键活化能。对于三铜物种的反应,我们建议使用过氧铜3O物种是中间结构,具有两个过渡态(TSs)参与反应。发现发生了三铜位点显着重排的第一个TS是速率决定的,而过氧键断裂的第二个TS则导致较小的活化障碍。与双铜的情况相反,该反应是轻微吸热的。本研究提供了理论上的见解,可能有助于设计更好的铜交换沸石催化剂,以甲烷甲烷化为甲醇。
更新日期:2018-08-09
中文翻译:

Cu-MOR沸石上的双氧活化:Cu 2 O和Cu 3 O 3活性物种形成的理论见解
在铜交换的沸石的活化中利用低成本和丰富的氧气(O 2)作为氧化剂对于在低温下将甲烷直接,选择性地氧化为甲醇非常重要。尽管在丝光沸石(MOR)中已通过实验观察到了活性位点的两个基序,即[Cu 2(μ-O)] 2+和[Cu 3(μ-O)3 ] 2+,但它们的机理Cu-MOR与O 2的反应形成的机理尚不清楚。在这项研究中,我们对2 [Cu 2 ] 2+ -MOR和[Cu 3 O]上的O 2活化进行了密度泛函理论(DFT)计算。2+ -MOR沸石。对于双铜物种的反应,我们发现了两种可能的反应途径:O-O键断裂导致(1)形成[Cu 2(μ-O)] 2+活性物种和反式-μ-1,2 -过氧-Si 2物种和(2)同时形成彼此相邻的两个[Cu 2(μ-O)] 2+活性物种。这些途径都是放热的,但需要完全不同的O–O键活化能。对于三铜物种的反应,我们建议使用过氧铜3O物种是中间结构,具有两个过渡态(TSs)参与反应。发现发生了三铜位点显着重排的第一个TS是速率决定的,而过氧键断裂的第二个TS则导致较小的活化障碍。与双铜的情况相反,该反应是轻微吸热的。本研究提供了理论上的见解,可能有助于设计更好的铜交换沸石催化剂,以甲烷甲烷化为甲醇。

































 京公网安备 11010802027423号
京公网安备 11010802027423号