当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 2-substituted 3-chlorobenzofurans via TMSCl-mediated nucleophilic annulation of isatin-derived propargylic alcohols
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-08-08 , DOI: 10.1039/c8ob01731j
Zhou Sun 1, 2, 3, 4 , Kuirong Xiang 1, 2, 3, 4 , Hua Tao 5, 6, 7 , Liqun Guo 1, 2, 3, 4 , Ying Li 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-08-08 , DOI: 10.1039/c8ob01731j
Zhou Sun 1, 2, 3, 4 , Kuirong Xiang 1, 2, 3, 4 , Hua Tao 5, 6, 7 , Liqun Guo 1, 2, 3, 4 , Ying Li 1, 2, 3, 4
Affiliation
|
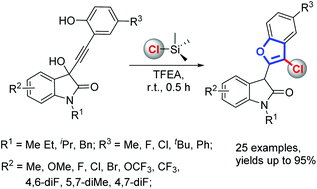
A TMSCl-mediated cascade annulation of isatin-derived propargylic alcohols for the synthesis of 2-substituted 3-chlorobenzofurans is now reported. Mechanistic investigations showed that this proceeded through a sequential Meyer–Schuster rearrangement/nucleophilic addition/intramolecular annulation. TMSCl not only acts as a promoter, but also acts as a chlorine source in this protocol.
中文翻译:

TMSC1介导的伊斯丁衍生的炔丙醇的亲核环化反应合成2-取代的3-氯苯并呋喃
现在报道了用于合成2-取代的3-氯苯并呋喃的由TMSA1介导的伊斯兰衍生的炔丙醇的级联环化。机理研究表明,这是通过依次进行Meyer-Schuster重排/亲核加成/分子内环化而进行的。在该方案中,TMSC1不仅充当启动子,而且充当氯源。
更新日期:2018-08-22
中文翻译:

TMSC1介导的伊斯丁衍生的炔丙醇的亲核环化反应合成2-取代的3-氯苯并呋喃
现在报道了用于合成2-取代的3-氯苯并呋喃的由TMSA1介导的伊斯兰衍生的炔丙醇的级联环化。机理研究表明,这是通过依次进行Meyer-Schuster重排/亲核加成/分子内环化而进行的。在该方案中,TMSC1不仅充当启动子,而且充当氯源。






































 京公网安备 11010802027423号
京公网安备 11010802027423号