当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-Catalytic Reaction of SO3 and NH3 to Produce Sulfamic Acid and Its Implication to Atmospheric Particle Formation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-08-08 , DOI: 10.1021/jacs.8b04928 Hao Li 1, 2 , Jie Zhong 2 , Hanna Vehkamäki , Theo Kurtén , Weigang Wang 3 , Maofa Ge 3 , Shaowen Zhang 1 , Zesheng Li 1 , Xiuhui Zhang 1 , Joseph S. Francisco 2 , Xiao Cheng Zeng 2, 4
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-08-08 , DOI: 10.1021/jacs.8b04928 Hao Li 1, 2 , Jie Zhong 2 , Hanna Vehkamäki , Theo Kurtén , Weigang Wang 3 , Maofa Ge 3 , Shaowen Zhang 1 , Zesheng Li 1 , Xiuhui Zhang 1 , Joseph S. Francisco 2 , Xiao Cheng Zeng 2, 4
Affiliation
|
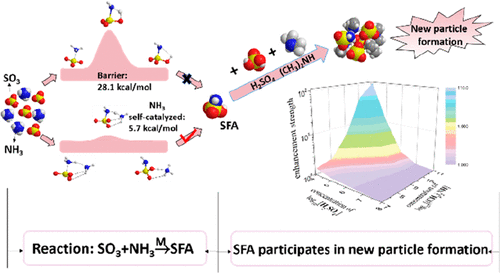
Sulfur trioxide (SO3) is one of the most active chemical species in the atmosphere, and its atmospheric fate has profound implications to air quality and human health. The dominant gas-phase loss pathway for SO3 is generally believed to be the reaction with water molecules, resulting in sulfuric acid. The latter is viewed as a critical component in the new particle formation (NPF). Herein, a new and competitive loss pathway for SO3 in the presence of abundant gas-phase ammonia (NH3) species is identified. Specifically, the reaction between SO3 and NH3, which produces sulfamic acid, can be self-catalyzed by the reactant (NH3). In dry and heavily polluted areas with relatively high concentrations of NH3, the effective rate constant for the bimolecular SO3-NH3 reaction can be sufficiently fast through this new loss pathway for SO3 to become competitive with the conventional loss pathway for SO3 with water. Furthermore, this study shows that the final product of the reaction, namely, sulfamic acid, can enhance the fastest possible rate of NPF from sulfuric acid and dimethylamine (DMA) by about a factor of 2. An alternative source of stabilizer for acid-base clustering in the atmosphere is suggested, and this new mechanism for NPF has potential to improve atmospheric modeling in highly polluted regions.
中文翻译:

SO3 和 NH3 自催化反应生成氨基磺酸及其对大气颗粒形成的影响
三氧化硫 (SO3) 是大气中最活跃的化学物质之一,其在大气中的归宿对空气质量和人类健康有着深远的影响。SO3 的主要气相损失途径通常被认为是与水分子反应,产生硫酸。后者被视为新粒子形成 (NPF) 的关键组成部分。在此,确定了存在大量气相氨 (NH3) 物质时 SO3 的新的竞争性损失途径。具体来说,SO3 和NH3 之间产生氨基磺酸的反应可以由反应物(NH3)自催化。在 NH3 浓度相对较高的干燥和重污染地区,通过这种新的 SO3 损失路径,双分子 SO3-NH3 反应的有效速率常数可以足够快,从而与 SO3 与水的常规损失路径竞争。此外,这项研究表明,反应的最终产物,即氨基磺酸,可以将硫酸和二甲胺 (DMA) 的 NPF 的最快速率提高约 2 倍。 酸碱稳定剂的替代来源建议在大气中进行聚类,这种 NPF 的新机制有可能改善高污染地区的大气建模。
更新日期:2018-08-08
中文翻译:

SO3 和 NH3 自催化反应生成氨基磺酸及其对大气颗粒形成的影响
三氧化硫 (SO3) 是大气中最活跃的化学物质之一,其在大气中的归宿对空气质量和人类健康有着深远的影响。SO3 的主要气相损失途径通常被认为是与水分子反应,产生硫酸。后者被视为新粒子形成 (NPF) 的关键组成部分。在此,确定了存在大量气相氨 (NH3) 物质时 SO3 的新的竞争性损失途径。具体来说,SO3 和NH3 之间产生氨基磺酸的反应可以由反应物(NH3)自催化。在 NH3 浓度相对较高的干燥和重污染地区,通过这种新的 SO3 损失路径,双分子 SO3-NH3 反应的有效速率常数可以足够快,从而与 SO3 与水的常规损失路径竞争。此外,这项研究表明,反应的最终产物,即氨基磺酸,可以将硫酸和二甲胺 (DMA) 的 NPF 的最快速率提高约 2 倍。 酸碱稳定剂的替代来源建议在大气中进行聚类,这种 NPF 的新机制有可能改善高污染地区的大气建模。













































 京公网安备 11010802027423号
京公网安备 11010802027423号