Nature Chemical Biology ( IF 12.9 ) Pub Date : 2015-12-07 , DOI: 10.1038/nchembio.1982 Michael B Battles 1 , Johannes P Langedijk 2 , Polina Furmanova-Hollenstein 2 , Supranee Chaiwatpongsakorn 3 , Heather M Costello 3 , Leen Kwanten 4 , Luc Vranckx 4 , Paul Vink 5 , Steffen Jaensch 5 , Tim H M Jonckers 6 , Anil Koul 4 , Eric Arnoult 7 , Mark E Peeples 3, 8 , Dirk Roymans 4 , Jason S McLellan 1
|
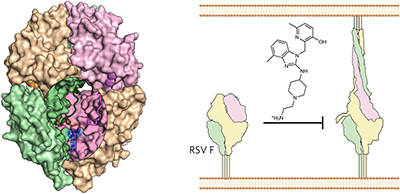
Respiratory syncytial virus (RSV) is a leading cause of pneumonia and bronchiolitis in young children and the elderly. Therapeutic small molecules have been developed that bind the RSV F glycoprotein and inhibit membrane fusion, yet their binding sites and molecular mechanisms of action remain largely unknown. Here we show that these inhibitors bind to a three-fold-symmetric pocket within the central cavity of the metastable prefusion conformation of RSV F. Inhibitor binding stabilizes this conformation by tethering two regions that must undergo a structural rearrangement to facilitate membrane fusion. Inhibitor-escape mutations occur in residues that directly contact the inhibitors or are involved in the conformational rearrangements required to accommodate inhibitor binding. Resistant viruses do not propagate as well as wild-type RSV in vitro, indicating a fitness cost for inhibitor escape. Collectively, these findings provide new insight into class I viral fusion proteins and should facilitate development of optimal RSV fusion inhibitors.
中文翻译:

呼吸道合胞病毒融合抑制剂的分子机制
呼吸道合胞病毒 (RSV) 是幼儿和老年人肺炎和细支气管炎的主要原因。已经开发出结合 RSV F 糖蛋白并抑制膜融合的治疗性小分子,但它们的结合位点和分子作用机制仍然很大程度上未知。在这里,我们证明这些抑制剂与 RSV F 亚稳态融合前构象中央腔内的三重对称口袋结合。抑制剂结合通过束缚必须经历结构重排以促进膜融合的两个区域来稳定该构象。抑制剂逃逸突变发生在直接接触抑制剂的残基中,或者参与适应抑制剂结合所需的构象重排。耐药病毒在体外的繁殖能力不如野生型 RSV,这表明抑制剂逃逸需要付出适应成本。总的来说,这些发现为 I 类病毒融合蛋白提供了新的见解,并应有助于开发最佳的 RSV 融合抑制剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号