当前位置:
X-MOL 学术
›
J. Am. Coll. Cardiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Erythrocytes From Patients With Type 2 Diabetes Induce Endothelial Dysfunction Via Arginase I
Journal of the American College of Cardiology ( IF 21.7 ) Pub Date : 2018-08-01 , DOI: 10.1016/j.jacc.2018.05.052 Zhichao Zhou 1 , Ali Mahdi 1 , Yahor Tratsiakovich 1 , Szabolcs Zahorán 2 , Oskar Kövamees 1 , Filip Nordin 1 , Arturo Eduardo Uribe Gonzalez 3 , Michael Alvarsson 4 , Claes-Göran Östenson 4 , Daniel C Andersson 5 , Ulf Hedin 6 , Edit Hermesz 2 , Jon O Lundberg 3 , Jiangning Yang 1 , John Pernow 7
Journal of the American College of Cardiology ( IF 21.7 ) Pub Date : 2018-08-01 , DOI: 10.1016/j.jacc.2018.05.052 Zhichao Zhou 1 , Ali Mahdi 1 , Yahor Tratsiakovich 1 , Szabolcs Zahorán 2 , Oskar Kövamees 1 , Filip Nordin 1 , Arturo Eduardo Uribe Gonzalez 3 , Michael Alvarsson 4 , Claes-Göran Östenson 4 , Daniel C Andersson 5 , Ulf Hedin 6 , Edit Hermesz 2 , Jon O Lundberg 3 , Jiangning Yang 1 , John Pernow 7
Affiliation
|
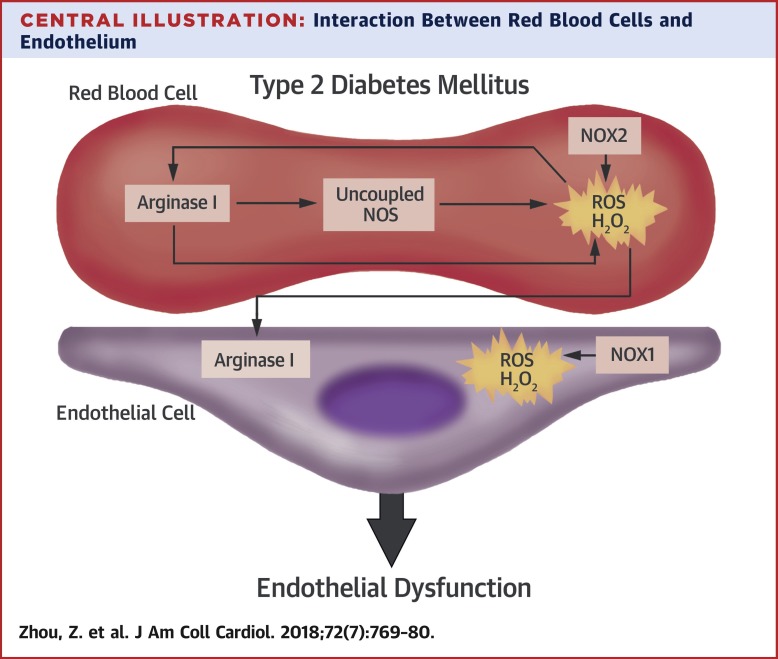
BACKGROUND
Cardiovascular complications are major clinical problems in type 2 diabetes mellitus (T2DM). The authors previously demonstrated a crucial role of red blood cells (RBCs) in control of cardiac function through arginase-dependent regulation of nitric oxide export from RBCs. There is alteration of RBC function, as well as an increase in arginase activity, in T2DM. OBJECTIVES
The authors hypothesized that RBCs from patients with T2DM induce endothelial dysfunction by up-regulation of arginase. METHODS
RBCs were isolated from patients with T2DM and age-matched healthy subjects and were incubated with rat aortas or human internal mammary arteries from nondiabetic patients for vascular reactivity and biochemical studies. RESULTS
Arginase activity and arginase I protein expression were elevated in RBCs from patients with T2DM (T2DM RBCs) through an effect induced by reactive oxygen species (ROS). Co-incubation of arterial segments with T2DM RBCs, but not RBCs from age-matched healthy subjects, significantly impaired endothelial function but not smooth muscle cell function in both healthy rat aortas and human internal mammary arteries. Endothelial dysfunction induced by T2DM RBCs was prevented by inhibition of arginase and ROS both at the RBC and vascular levels. T2DM RBCs induced increased vascular arginase I expression and activity through an ROS-dependent mechanism. CONCLUSIONS
This study demonstrates a novel mechanism behind endothelial dysfunction in T2DM that is induced by RBC arginase I and ROS. Targeting arginase I in RBCs may serve as a novel therapeutic tool for the treatment of endothelial dysfunction in T2DM.
中文翻译:

2 型糖尿病患者的红细胞通过精氨酸酶 I 诱导内皮功能障碍
背景心血管并发症是2型糖尿病(T2DM)的主要临床问题。作者先前证明了红细胞 (RBC) 在通过精氨酸酶依赖性调节 RBC 的一氧化氮输出来控制心脏功能中的关键作用。在 T2DM 中,红细胞功能发生改变,精氨酸酶活性增加。目的 作者假设 T2DM 患者的红细胞通过上调精氨酸酶诱导内皮功能障碍。方法 从 T2DM 患者和年龄匹配的健康受试者中分离出红细胞,并与来自非糖尿病患者的大鼠主动脉或人内乳动脉一起孵育,用于血管反应性和生化研究。结果 通过活性氧 (ROS) 诱导的作用,T2DM 患者的红细胞 (T2DM RBC) 中的精氨酸酶活性和精氨酸酶 I 蛋白表达升高。动脉节段与 T2DM 红细胞的共同培养,而不是来自年龄匹配的健康受试者的红细胞,显着损害健康大鼠主动脉和人内乳动脉的内皮功能,但不损害平滑肌细胞功能。通过在 RBC 和血管水平抑制精氨酸酶和 ROS,可以防止由 T2DM 红细胞诱导的内皮功能障碍。T2DM 红细胞通过 ROS 依赖性机制诱导血管精氨酸酶 I 表达和活性增加。结论 本研究证明了由红细胞精氨酸酶 I 和 ROS 诱导的 T2DM 内皮功能障碍背后的新机制。
更新日期:2018-08-01
中文翻译:

2 型糖尿病患者的红细胞通过精氨酸酶 I 诱导内皮功能障碍
背景心血管并发症是2型糖尿病(T2DM)的主要临床问题。作者先前证明了红细胞 (RBC) 在通过精氨酸酶依赖性调节 RBC 的一氧化氮输出来控制心脏功能中的关键作用。在 T2DM 中,红细胞功能发生改变,精氨酸酶活性增加。目的 作者假设 T2DM 患者的红细胞通过上调精氨酸酶诱导内皮功能障碍。方法 从 T2DM 患者和年龄匹配的健康受试者中分离出红细胞,并与来自非糖尿病患者的大鼠主动脉或人内乳动脉一起孵育,用于血管反应性和生化研究。结果 通过活性氧 (ROS) 诱导的作用,T2DM 患者的红细胞 (T2DM RBC) 中的精氨酸酶活性和精氨酸酶 I 蛋白表达升高。动脉节段与 T2DM 红细胞的共同培养,而不是来自年龄匹配的健康受试者的红细胞,显着损害健康大鼠主动脉和人内乳动脉的内皮功能,但不损害平滑肌细胞功能。通过在 RBC 和血管水平抑制精氨酸酶和 ROS,可以防止由 T2DM 红细胞诱导的内皮功能障碍。T2DM 红细胞通过 ROS 依赖性机制诱导血管精氨酸酶 I 表达和活性增加。结论 本研究证明了由红细胞精氨酸酶 I 和 ROS 诱导的 T2DM 内皮功能障碍背后的新机制。















































 京公网安备 11010802027423号
京公网安备 11010802027423号