当前位置:
X-MOL 学术
›
Adv. Opt. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Plasmonic Nanoprobes for Multiplexed Fluorescence‐Free Super‐Resolution Imaging
Advanced Optical Materials ( IF 8.0 ) Pub Date : 2018-08-05 , DOI: 10.1002/adom.201800432 Jian Xu 1 , Tianyue Zhang 1 , Shenyu Yang 2 , Ziwei Feng 1 , Haoying Li 2 , Dejiao Hu 1 , Fei Qin 1 , Xueying Ouyang 1 , Yaoyu Cao 1 , Lingxiang Jiang 2 , Xiangping Li 1
Advanced Optical Materials ( IF 8.0 ) Pub Date : 2018-08-05 , DOI: 10.1002/adom.201800432 Jian Xu 1 , Tianyue Zhang 1 , Shenyu Yang 2 , Ziwei Feng 1 , Haoying Li 2 , Dejiao Hu 1 , Fei Qin 1 , Xueying Ouyang 1 , Yaoyu Cao 1 , Lingxiang Jiang 2 , Xiangping Li 1
Affiliation
|
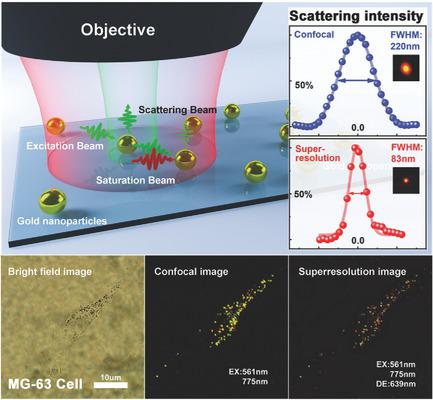
Noble metal nanoparticles (NPs), owing to their unique optical and physicochemical properties, are routinely used for optical imaging and labeling of biological specimens. Even though they can provide vital information for studying multiple cellular events and their interplays at the same time, optically multiplexing and resolving specific NPs within a diffraction‐limited region labeled in complex biological specimens remains a fundamental challenge. By introducing and manipulating plasmonic resonance assisted saturable scattering effects, multiplexed fluorescence‐free super‐resolution imaging of gold NPs in tumor cells with remarkable subdiffraction resolution is demonstrated. The saturable scattering allows fluorescence‐free resolving single plasmonic nanoprobes with significantly improved resolution down to ≈100 nm. The revealed plasmonic resonance assisted saturation effect as well as the associated spectral flexibility to variant sizes provides access to multiplexing capability in complex bioenvironments. The demonstrated feasibility of two‐color super‐resolution cellular imaging is achieved at ultralow suppression powers ≈0.28 MW cm−2, corresponding to a two‐order of magnitude improvement compared to the state‐of‐the‐art of stimulated emission depletion (STED) nanoscopy.
中文翻译:

等离子纳米探针用于无荧光多通道超高分辨率成像
贵金属纳米颗粒(NPs)由于其独特的光学和物理化学特性,通常用于生物标本的光学成像和标记。尽管它们可以为同时研究多个细胞事件及其相互作用提供重要信息,但是在复杂生物样品中标记的衍射受限区域内光学复用和解析特定NP仍然是一项基本挑战。通过引入和操纵等离振子共振辅助的饱和散射效应,展示了具有显着亚衍射分辨率的肿瘤细胞中金纳米颗粒的无荧光多路复用多重成像。饱和散射可实现无荧光的单等离子体纳米探针的分离,分辨率低至≈100nm。揭示的等离激元共振辅助饱和效应以及相关的变体尺寸光谱灵活性为复杂生物环境中的多路复用能力提供了途径。在超低抑制功率≈0.28MW cm下实现了双色超分辨率细胞成像的可行性−2,与最新的受激发射损耗(STED)纳米显微镜相比,提高了两个数量级。
更新日期:2018-08-05
中文翻译:

等离子纳米探针用于无荧光多通道超高分辨率成像
贵金属纳米颗粒(NPs)由于其独特的光学和物理化学特性,通常用于生物标本的光学成像和标记。尽管它们可以为同时研究多个细胞事件及其相互作用提供重要信息,但是在复杂生物样品中标记的衍射受限区域内光学复用和解析特定NP仍然是一项基本挑战。通过引入和操纵等离振子共振辅助的饱和散射效应,展示了具有显着亚衍射分辨率的肿瘤细胞中金纳米颗粒的无荧光多路复用多重成像。饱和散射可实现无荧光的单等离子体纳米探针的分离,分辨率低至≈100nm。揭示的等离激元共振辅助饱和效应以及相关的变体尺寸光谱灵活性为复杂生物环境中的多路复用能力提供了途径。在超低抑制功率≈0.28MW cm下实现了双色超分辨率细胞成像的可行性−2,与最新的受激发射损耗(STED)纳米显微镜相比,提高了两个数量级。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号