当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fe3O4 @ PLGA‐PEG Nanocomposite for Improved Delivery of Methotrexate in Cancer Treatment
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-08-06 , DOI: 10.1002/slct.201801769 Tanushree Basu 1 , Satnam Singh 1 , Bonamali Pal 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-08-06 , DOI: 10.1002/slct.201801769 Tanushree Basu 1 , Satnam Singh 1 , Bonamali Pal 1
Affiliation
|
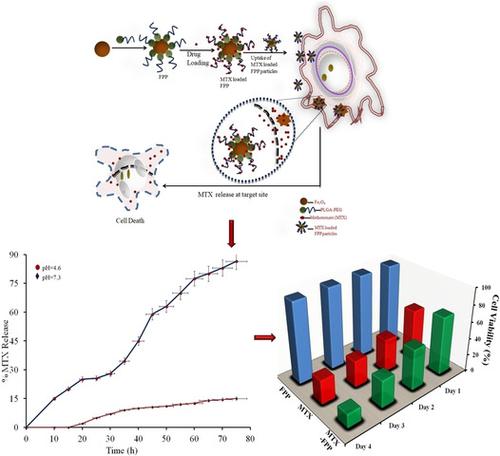
Magnetic Fe3O4 nanoparticles are gaining significance in drug delivery applications owing to their targeting capability. Surface modification of amphiphilic block polymers by Fe3O4 nanoparticles increases their properties. In this study, Fe3O4 @ PLGA‐PEG nanocomposite is prepared by double emulsion (w/o/w) method. A shift in the 2Θ values for the composite in XRD attributes to interaction between Fe3O4 and PLGA‐PEG. Also, a shift in the Fe‐O band in the FTIR spectrum of Fe3O4@PLGA‐PEG from 578 cm‐1 to 510 cm‐1 confirms the formation of nanocomposite. Surface morphology of the prepared nanocomposite is analyzed by TEM and AFM. Decrease in agglomeration due to electrostatic repulsion between the polymer chains and magnetic particles is observed while an increased surface area (61.0nm) confirms the formation of the nanocomposite. To determine the effectiveness of the prepared magnetically modified nanoparticles, methotrexate (anticancer drug) is encapsulated into the nanocomposite. High entrapment efficiency of 95% is observed when polymer:drug is 1:1. The in‐vitro release profile shows that pH of release medium plays a significant role. At physiological pH of 7.3 there is only 15% methotrexate release while nearly 86% of methotrexate release is observed at acidic pH of 4.6 over 72h. Korsemeyer‐Peppas model of drug release (R2‐0.9868) represents swelling controlled release of methotrexate. Further, the cytotoxic cell viability assay on SK‐BR‐3 (breast adinocarcinoma) cells showed that methotrexate loaded onto the nanocomposite showed higher cell viability as compared to free methotrexate after 96h of incubation. The fluorescent cell imaging also showed that methotrexate released slowly from the nanoparticles and diffused into the nucleus without losing its cytotoxic effect on the cancer cells. Based on these properties of the magnetically modified PLGA‐PEG nanoparticles they can be used as targeting drug delivery agents in treatment of cancer therapy.
中文翻译:

Fe3O4 @ PLGA-PEG纳米复合材料可改善甲氨蝶呤在癌症治疗中的递送
磁性Fe 3 O 4纳米粒子由于其靶向能力而在药物递送应用中变得越来越重要。Fe 3 O 4纳米颗粒对两亲性嵌段聚合物进行表面改性可提高其性能。在这项研究中,Fe 3 O 4 @ PLGA-PEG纳米复合材料是通过双重乳液(w / o / w)方法制备的。复合材料在XRD中2θ值的变化归因于Fe 3 O 4与PLGA-PEG之间的相互作用。另外,Fe 3 O 4 @ PLGA-PEG的FTIR光谱中的Fe-O谱带从578 cm -1转变为510 cm -1证实了纳米复合材料的形成。通过TEM和AFM分析制备的纳米复合材料的表面形态。观察到由于聚合物链和磁性颗粒之间的静电排斥而导致的团聚减少,而增加的表面积(61.0nm)证实了纳米复合材料的形成。为了确定所制备的磁性改性纳米粒子的有效性,将甲氨蝶呤(抗癌药)封装到纳米复合材料中。当聚合物:药物为1∶1时,观察到95%的高包封率。在体外释放曲线表明,释放介质的pH值起着重要作用。在7.3的生理pH下,只有72%的酸性pH值为4.6,而氨甲蝶呤的释放率为15%,而在4.6的酸性pH下,氨甲蝶呤的释放率为86%。Korsemeyer-Peppas药物释放模型(R 2-0.9868)代表甲氨蝶呤的溶胀控制释放。此外,对SK‐BR-3(乳腺癌)的细胞进行的细胞毒性细胞活力测定表明,与96小时孵育后的游离甲氨蝶呤相比,负载在纳米复合材料上的甲氨蝶呤显示出更高的细胞活力。荧光细胞成像还显示甲氨蝶呤从纳米颗粒缓慢释放并扩散到细胞核中,而不会失去其对癌细胞的细胞毒性作用。基于磁性修饰的PLGA-PEG纳米粒子的这些特性,它们可用作靶向药物递送剂,用于治疗癌症。
更新日期:2018-08-06
中文翻译:

Fe3O4 @ PLGA-PEG纳米复合材料可改善甲氨蝶呤在癌症治疗中的递送
磁性Fe 3 O 4纳米粒子由于其靶向能力而在药物递送应用中变得越来越重要。Fe 3 O 4纳米颗粒对两亲性嵌段聚合物进行表面改性可提高其性能。在这项研究中,Fe 3 O 4 @ PLGA-PEG纳米复合材料是通过双重乳液(w / o / w)方法制备的。复合材料在XRD中2θ值的变化归因于Fe 3 O 4与PLGA-PEG之间的相互作用。另外,Fe 3 O 4 @ PLGA-PEG的FTIR光谱中的Fe-O谱带从578 cm -1转变为510 cm -1证实了纳米复合材料的形成。通过TEM和AFM分析制备的纳米复合材料的表面形态。观察到由于聚合物链和磁性颗粒之间的静电排斥而导致的团聚减少,而增加的表面积(61.0nm)证实了纳米复合材料的形成。为了确定所制备的磁性改性纳米粒子的有效性,将甲氨蝶呤(抗癌药)封装到纳米复合材料中。当聚合物:药物为1∶1时,观察到95%的高包封率。在体外释放曲线表明,释放介质的pH值起着重要作用。在7.3的生理pH下,只有72%的酸性pH值为4.6,而氨甲蝶呤的释放率为15%,而在4.6的酸性pH下,氨甲蝶呤的释放率为86%。Korsemeyer-Peppas药物释放模型(R 2-0.9868)代表甲氨蝶呤的溶胀控制释放。此外,对SK‐BR-3(乳腺癌)的细胞进行的细胞毒性细胞活力测定表明,与96小时孵育后的游离甲氨蝶呤相比,负载在纳米复合材料上的甲氨蝶呤显示出更高的细胞活力。荧光细胞成像还显示甲氨蝶呤从纳米颗粒缓慢释放并扩散到细胞核中,而不会失去其对癌细胞的细胞毒性作用。基于磁性修饰的PLGA-PEG纳米粒子的这些特性,它们可用作靶向药物递送剂,用于治疗癌症。















































 京公网安备 11010802027423号
京公网安备 11010802027423号