European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2018-08-04 , DOI: 10.1016/j.ejmech.2018.08.003 Robert Zscherp , Sören Baumeister , Dirk Schepmann , Bernhard Wünsch
|
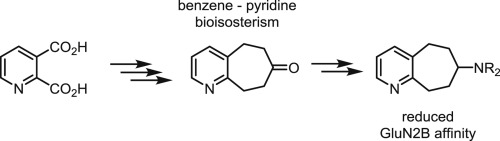
It has been reported that benzo [7]annulen-7-amines bearing electron withdrawing substituents such as 3d with a 2-Cl or 3e with a 2-NO2 moiety show very high affinity towards the ifenprodil binding site of GluN2B subunit containing NMDA receptors. Therefore, bioisosteres of 3 with an electron deficient pyridine ring instead of the chloro- or nitrobenzene ring were envisaged. Starting from pyridine-2,3-dicarboxylic acid (5) a five-step synthesis of the key intermediate, the ketone 10, was developed. Reductive amination with various primary amines and NaBH(OAc)3 led to the homologous secondary amines 11a-c. Subsequent methylation yielded the tertiary amines 12b and 12c. Receptor binding studies with [3H]ifenprodil revealed Ki-values above 100 nM for the most active phenylpropyl- and phenylbutylamines 11b and 11c. The >100-fold reduced GluN2B affinity of pyridines 11b and 11c compared to the GluN2B affinity of the corresponding chloro- and nitrobenzene derivatives 3d and 3e indicates that the pyridine ring is not tolerated as bioisosteric replacement of the chloro- or nitrobenzene ring in this type of compounds.
中文翻译:

含有NMDA受体拮抗剂的强效GluN2B亚基与苯并[7]环戊烯基吡啶的吡啶生物同工异构体
据报道,带有吸电子取代基的苯并[7]环烯-7-胺,例如带有2-Cl或带有2-NO 2部分的3e的3d,对含有NMDA受体的GluN2B亚基的艾芬地尔结合位点显示出很高的亲和力。 。因此,设想了具有电子不足的吡啶环而不是氯或硝基苯环的3的生物等排体。从吡啶-2,3-二羧酸(5)开始,开发了关键中间体酮10的五步合成法。与各种伯胺和NaBH(OAc)3的还原胺化导致同源仲胺11a - c。随后的甲基化产生叔胺12b和12c。用[ 3 H] ifenprodil进行的受体结合研究表明,对于最具活性的苯基丙基胺和苯基丁基胺11b和11c,其K i值高于100 nM 。与相应的氯苯和硝基苯衍生物3d和3e的GluN2B亲和力相比,吡啶11b和11c的GluN2B亲和力降低100倍以上,表明这种环不耐受吡啶环作为氯代或硝基苯环的生物等位取代化合物。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号