Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2018-08-03 , DOI: 10.1016/j.jinorgbio.2018.08.005 Yue Zhang , Lichao Wang , Kewu Zeng , Kui Wang , Xiaoda Yang
|
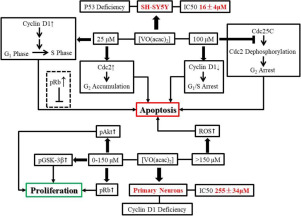
Vanadium compounds have arisen as potential therapeutic agent for the treatment of cancers over the past decades. A few studies suggested that vanadyl complexes may discriminate between the cancerous and the normal cells. Here, we reported the investigation on the pro-apoptotic effect and the underlying mechanism of bis(acetylacetonato) oxovanadium(IV) ([VO(acac)2]) on SH-SY5Y neuroblastoma cells in comparison with that of mouse primary cortex neurons. The experimental results revealed that [VO(acac)2] showed about 10-fold higher cytotoxicity (IC50 ~16 μM) on the neuroblastoma cells than on normal neurons (IC50 ~250 μM). Further analysis indicated that the vanadyl complex suppressed the growth of neuroblastoma cells via different pathways depending on its concentration. It induced a special cyclin D-mediated and p53-independent cell apoptosis at <50 μM but cell cycle arrests at >50 μM. In contrast, [VO(acac)2] promoted cell viability of primary neurons in the concentration range of 0–150 μM; while [VO(acac)2] at hundreds of μM would cause neuronal death possibly via the reactive oxygen species (ROS)-mediated signal pathways. The extraordinary discrimination between neuroblastoma cells and primary neurons suggests potential application of vanadyl complexes for therapeutic treatment of neuroblastoma. In addition, the p53-independent apoptotic pathways induced by vanadyl complexes may provide new insights for future discovery of new anticancer drugs overcoming the chemo-resistance due to p53 mutation.
中文翻译:

钒基复合物通过诱导细胞特异性凋亡途径来区分神经母细胞瘤细胞和原代神经元
在过去的几十年中,钒化合物已经成为治疗癌症的潜在治疗剂。一些研究表明,钒基复合物可以区分癌细胞和正常细胞。在这里,我们报道了与小鼠原代皮层神经元相比,双(乙酰丙酮基)氧钒(IV)([VO(acac)2 ])对SH-SY5Y神经母细胞瘤细胞的促凋亡作用及其潜在机制的研究。实验结果表明,[VO(acac)2]显示出对神经母细胞瘤细胞的细胞毒性(IC50〜16μM)比对正常神经元(IC50〜250μM)高约10倍。进一步的分析表明,钒基复合物根据其浓度通过不同途径抑制神经母细胞瘤细胞的生长。它在<50μM时诱导了特殊的细胞周期蛋白D介导且不依赖p53的细胞凋亡,但在> 50μM时细胞周期停滞。相反,[VO(acac)2 ]可以在0-150μM的浓度范围内促进原代神经元的细胞活力。而[VO(acac)2]浓度为数百μM时,可能会通过活性氧(ROS)介导的信号通路导致神经元死亡。神经母细胞瘤细胞和原代神经元之间的非同寻常的区别表明,钒基复合物在神经母细胞瘤的治疗中具有潜在的应用前景。此外,钒基复合物诱导的非p53凋亡途径可能为将来发现克服因p53突变而引起的化学耐药性的新抗癌药物提供新的见解。































 京公网安备 11010802027423号
京公网安备 11010802027423号