European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2018-08-02 , DOI: 10.1016/j.ejmech.2018.07.065 Cristina Pérez-Arnaiz , María Isabel Acuña , Natalia Busto , Igor Echevarría , Marta Martínez-Alonso , Gustavo Espino , Begoña García , Fernando Domínguez
|
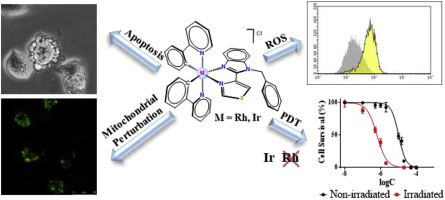
Two pairs of Rh(III) and Ir(III) biscyclometallated complexes with thiabendazole (L1), named [Ir-a]Cl and [Rh-a]Cl, and N-benzyl-thiabendazole (L2), named [Ir-b]Cl and [Rh-b]Cl, have been designed and synthesized to explore the photophysical and biological effects that arise from changing both the metal center and the ancillary ligand. In the dark, the four metal complexes exhibit greater cytotoxicity than cisplatin against human colon (SW480) and human lung (A549) adenocarcinoma cell lines. Moreover, the pair of complexes bearing the ligand L2 is markedly more cytotoxic and present higher uptake values than complexes with L1, thereby their biological properties were studied further to determine their mechanism of action. Interestingly, in spite of the different metal center both the [Ir-b]Cl and [Rh-b]Cl complexes are responsible for the loss of mitochondrial functionality and the activation of apoptotic cell death pathways. Moreover, the photodynamic activity of the four complexes, [Ir-a,b]Cl and [Rh-a,b]Cl, was tested using visible blue light (460 nm) under soft irradiation conditions (20 min, 5.5 mW cm−2). While the Rh complexes are not photopotentiated, the phototoxicity index (IC50 non-irradiated/IC50 irradiated) of [Ir-a]Cl and [Ir-b]Cl complexes was 15.8 and 3.6, respectively. We also demonstrate that only the Ir derivatives are capable of photocatalyzing the oxidation of S-containing l-amino acids under blue light irradiation, [Ir-a]Cl being more active than [Ir-b]Cl, which provides a reasonable mechanism for their biological action (oxidative stress could be selectively promoted through a photocatalytic action) upon irradiation. This different PDT behaviour depending on the metal center and the ancillary substituent may be useful for future rational design of metal-based photosensitizers.
中文翻译:

基于噻苯达唑的Rh(III)和Ir(III)双环金属化配合物,具有线粒体靶向的抗癌活性和对金属敏感的光动力学活性
两对Rh(III)和Ir(III)与噻苯达唑(L 1)的双环金属化配合物,分别命名为[Ir-a] Cl和[Rh-a] Cl和N-苄基-噻苯达唑(L 2),命名为[Ir -b] Cl和[Rh-b] Cl已被设计和合成,以探索由于改变金属中心和辅助配体而产生的光物理和生物效应。在黑暗中,四种金属配合物对人结肠癌(SW480)和人肺腺癌(A549)的细胞毒性要比顺铂高。而且,与具有L 2的配合物相比,带有配体L 2的配合物对具有更高的细胞毒性并具有更高的摄取值。参照图1,因此对其生物学特性进行了进一步研究以确定它们的作用机理。有趣的是,尽管金属中心不同,但[Ir-b] Cl和[Rh-b] Cl络合物都负责线粒体功能的丧失和凋亡细胞死亡途径的激活。此外,四个复合物的光动力活性,物[Ir-A,B]氯和铑[Rh-A,B]氯,是软的照射条件下,用蓝色可见光(460纳米)(测试20分钟,5.5毫瓦厘米- 2)。而上的Rh络合物不photopotentiated,光毒性指数(IC 50未照射/ IC 50的照射)的[Ir-A]氯和[Ir-b] Cl配合物分别为15.8和3.6。我们还证明,只有Ir衍生物才能在蓝光照射下光催化含S的1-氨基酸的氧化,[Ir-a] Cl比[Ir-b] Cl更具活性,这为它们在辐射下的生物学作用(可以通过光催化作用选择性地促进氧化应激)。取决于金属中心和辅助取代基的这种不同的PDT行为可能对将来基于金属的光敏剂的合理设计有用。































 京公网安备 11010802027423号
京公网安备 11010802027423号