当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synergy between Zeolite Framework and Encapsulated Sulfur for Enhanced Ion-Exchange Selectivity to Radioactive Cesium
Chemistry of Materials ( IF 7.2 ) Pub Date : 2018-08-02 00:00:00 , DOI: 10.1021/acs.chemmater.8b02782 Eunhye Han 1 , Young-Gu Kim 1 , Hee-Man Yang 2 , In-Ho Yoon 2 , Minkee Choi 1
Chemistry of Materials ( IF 7.2 ) Pub Date : 2018-08-02 00:00:00 , DOI: 10.1021/acs.chemmater.8b02782 Eunhye Han 1 , Young-Gu Kim 1 , Hee-Man Yang 2 , In-Ho Yoon 2 , Minkee Choi 1
Affiliation
|
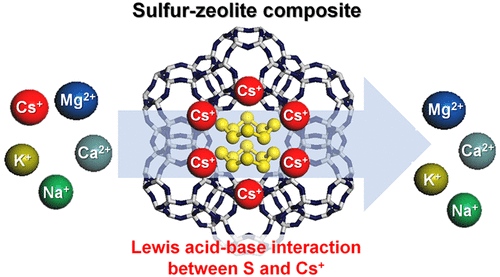
To eliminate the radioisotope 137Cs+ from contaminated water, various inorganic ion-exchange materials have been developed. Many selective ion-exchange materials are relatively expensive and difficult to prepare, whereas conventional materials such as aluminosilicate zeolites lack ion-exchange selectivity in the presence of competing cations. Here, we report a simple but powerful strategy to significantly increase the Cs+ selectivity of conventional zeolites. We demonstrate that encapsulation of elemental sulfur in the micropores of zeolites (NaA, NaX, chabazite, and mordenite) via vacuum sublimation can remarkably increase the selectivity toward Cs+ in the presence of competing ions. It appears that the elemental sulfur does not provide additional adsorption sites for Cs+ ions but increases the ion-exchange selectivity toward Cs+ by providing additional interaction. Various analyses show that sulfur partially donates its electron to the ion-exchanged Cs+ cations in zeolites, indicating significant Lewis acid–base interaction. According to the hard soft acid base (HSAB) theory, the enhanced Cs+ ion-exchange selectivity can be explained by the fact that sulfur, a soft Lewis base, interacts more strongly with Cs+, which is a softer Lewis acid than other alkali and alkaline earth metal cations. Because of the high intrinsic Cs+ selectivity of bare zeolites and selectivity enhancement resulting from sulfur encapsulation, the sulfur-modified chabazite and mordenite showed highly promising Cs+ capture ability in the presence of various competing ions.
中文翻译:

沸石骨架与封装硫之间的协同作用,以增强对放射性铯的离子交换选择性
为了从污染的水中消除放射性同位素137 Cs +,已经开发了各种无机离子交换材料。许多选择性离子交换材料相对昂贵且难以制备,而常规材料(例如铝硅酸盐沸石)在存在竞争性阳离子的情况下缺乏离子交换选择性。在这里,我们报告了一种简单而有效的策略,可以显着提高常规沸石的Cs +选择性。我们证明了通过真空升华将分子硫包裹在沸石(NaA,NaX,菱沸石和丝光沸石)的微孔中可以显着提高对Cs +的选择性在存在竞争离子的情况下。看来,元素硫不针对CS提供额外的吸附位点+离子,但增加向Cs中的离子交换选择性+通过提供附加的交互。各种分析表明,硫将其电子部分提供给沸石中离子交换的Cs +阳离子,这表明路易斯酸与碱之间存在显着相互作用。根据硬软酸碱(HSAB)理论,增强的铯+离子交换选择性,可以通过以下事实解释,硫,软路易斯碱相互作用更强烈铯+ ,这是一种较软的路易斯酸以外的碱和碱土金属阳离子。由于固有Cs +高裸露沸石的选择性和硫包封,硫改性菱沸石和丝光沸石的选择性增强在各种竞争性离子存在下均显示出极有希望的Cs +捕获能力。
更新日期:2018-08-02
中文翻译:

沸石骨架与封装硫之间的协同作用,以增强对放射性铯的离子交换选择性
为了从污染的水中消除放射性同位素137 Cs +,已经开发了各种无机离子交换材料。许多选择性离子交换材料相对昂贵且难以制备,而常规材料(例如铝硅酸盐沸石)在存在竞争性阳离子的情况下缺乏离子交换选择性。在这里,我们报告了一种简单而有效的策略,可以显着提高常规沸石的Cs +选择性。我们证明了通过真空升华将分子硫包裹在沸石(NaA,NaX,菱沸石和丝光沸石)的微孔中可以显着提高对Cs +的选择性在存在竞争离子的情况下。看来,元素硫不针对CS提供额外的吸附位点+离子,但增加向Cs中的离子交换选择性+通过提供附加的交互。各种分析表明,硫将其电子部分提供给沸石中离子交换的Cs +阳离子,这表明路易斯酸与碱之间存在显着相互作用。根据硬软酸碱(HSAB)理论,增强的铯+离子交换选择性,可以通过以下事实解释,硫,软路易斯碱相互作用更强烈铯+ ,这是一种较软的路易斯酸以外的碱和碱土金属阳离子。由于固有Cs +高裸露沸石的选择性和硫包封,硫改性菱沸石和丝光沸石的选择性增强在各种竞争性离子存在下均显示出极有希望的Cs +捕获能力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号