当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
cAMP, Ca2+, pHi, and NO Regulate H-like Cation Channels That Underlie Feeding and Locomotion in the Predatory Sea Slug Pleurobranchaea californica
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-08-01 00:00:00 , DOI: 10.1021/acschemneuro.8b00187 Daniel J Green 1 , Rong-Chi Huang 2 , Leland Sudlow 2 , Nathan Hatcher 2 , Kurt Potgieter 2 , Catherine McCrohan 3 , Colin Lee 1 , Elena V Romanova 4 , Jonathan V Sweedler 4 , Martha L U Gillette 5 , Rhanor Gillette 2
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-08-01 00:00:00 , DOI: 10.1021/acschemneuro.8b00187 Daniel J Green 1 , Rong-Chi Huang 2 , Leland Sudlow 2 , Nathan Hatcher 2 , Kurt Potgieter 2 , Catherine McCrohan 3 , Colin Lee 1 , Elena V Romanova 4 , Jonathan V Sweedler 4 , Martha L U Gillette 5 , Rhanor Gillette 2
Affiliation
|
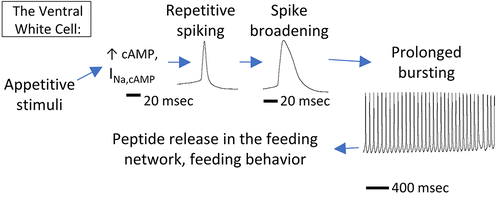
A systems approach to regulation of neuronal excitation in the mollusc Pleurobranchaea has described novel interactions of cyclic AMP-gated cation current (INa,cAMP), Ca2+, pHi, and NO. INa,cAMP appears in many neurons of feeding and locomotor neuronal networks. It is likely one of the family of hyperpolarization-activated, cyclic-nucleotide-gated currents (h-current) of vertebrate and invertebrate pacemaker networks. There are two isoforms. Ca2+ regulates both voltage dependence and depolarization-sensitive inactivation in both isoforms. The Type 1 INa,cAMP of the feeding network is enhanced by intracellular acidification. A direct dependence of INa,cAMP on cAMP allows the current to be used as a reporter on cAMP concentrations in the cell, and from there to the intrinsic activities of the synthetic adenyl cyclase and the degradative phosphodiesterase. Type 2 INa,cAMP of the locomotor system is activated by serotonergic inputs, while Type 1 of the feeding network is thought to be regulated peptidergically. NO synthase activity is high in the CNS, where it differs from standard neuronal NO synthase in not being Ca2+ sensitive. NO acidifies pHi, potentiating Type 1, and may act to open proton channels. A cGMP pathway does not mediate NO effects as in other systems. Rather, nitrosylation likely mediates its actions. An integrated model of the action of cAMP, Ca2+, pHi, and NO in the feeding network postulates that NO regulates proton conductance to cause neuronal excitation in the cell body on the one hand, and relief of activity-induced hyperacidification in fine dendritic processes on the other.
中文翻译:

cAMP、Ca2+、pHi 和 NO 调节 H 样阳离子通道,这是捕食性海蛞蝓 Pleurobranchaea californica 摄食和运动的基础
一种调节软体动物胸鳃动物神经元兴奋的系统方法描述了环状 AMP 门控阳离子电流 (INa,cAMP)、Ca2+、pH 和 NO 的新相互作用。INa,cAMP 出现在摄食和运动神经元网络的许多神经元中。它可能是脊椎动物和无脊椎动物起搏器网络的超极化激活的环核苷酸门控电流 (h-current) 家族之一。有两种亚型。Ca2+ 调节两种亚型中的电压依赖性和去极化敏感失活。饲养网络的 1 型I Na,cAMP 因细胞内酸化而增强。INa,cAMP 对 cAMP 的直接依赖性允许电流用作细胞中 cAMP 浓度的报告基因,并从那里到合成腺苷酸环化酶和降解磷酸二酯酶的内在活性。运动系统的 2 型I Na,cAMP 被血清素能输入激活,而 1 型摄食网络被认为受到肽酶调节。NO 合酶活性在 CNS 中很高,它与标准神经元 NO 合酶的不同之处在于对 Ca2+ 不敏感。NO 酸化 pH 值,增强 1 型,并可能打开质子通道。cGMP 通路不像其他系统那样介导 NO 效应。相反,亚硝基化可能介导其作用。cAMP、Ca2+、pH 和 NO 在饲养网络中的作用的综合模型假设 NO 一方面调节质子电导以引起细胞体内的神经元兴奋,另一方面缓解细树突过程中活动诱导的过度酸化。
更新日期:2018-08-01
中文翻译:

cAMP、Ca2+、pHi 和 NO 调节 H 样阳离子通道,这是捕食性海蛞蝓 Pleurobranchaea californica 摄食和运动的基础
一种调节软体动物胸鳃动物神经元兴奋的系统方法描述了环状 AMP 门控阳离子电流 (INa,cAMP)、Ca2+、pH 和 NO 的新相互作用。INa,cAMP 出现在摄食和运动神经元网络的许多神经元中。它可能是脊椎动物和无脊椎动物起搏器网络的超极化激活的环核苷酸门控电流 (h-current) 家族之一。有两种亚型。Ca2+ 调节两种亚型中的电压依赖性和去极化敏感失活。饲养网络的 1 型I Na,cAMP 因细胞内酸化而增强。INa,cAMP 对 cAMP 的直接依赖性允许电流用作细胞中 cAMP 浓度的报告基因,并从那里到合成腺苷酸环化酶和降解磷酸二酯酶的内在活性。运动系统的 2 型I Na,cAMP 被血清素能输入激活,而 1 型摄食网络被认为受到肽酶调节。NO 合酶活性在 CNS 中很高,它与标准神经元 NO 合酶的不同之处在于对 Ca2+ 不敏感。NO 酸化 pH 值,增强 1 型,并可能打开质子通道。cGMP 通路不像其他系统那样介导 NO 效应。相反,亚硝基化可能介导其作用。cAMP、Ca2+、pH 和 NO 在饲养网络中的作用的综合模型假设 NO 一方面调节质子电导以引起细胞体内的神经元兴奋,另一方面缓解细树突过程中活动诱导的过度酸化。

































 京公网安备 11010802027423号
京公网安备 11010802027423号