当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Redox Conversion of Arsenite and Nitrate in the UV/Quinone Systems
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-08-14 , DOI: 10.1021/acs.est.8b03538 Zhihao Chen 1 , Jiyuan Jin 1 , Xiaojie Song 1 , Guoyang Zhang 1 , Shujuan Zhang 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-08-14 , DOI: 10.1021/acs.est.8b03538 Zhihao Chen 1 , Jiyuan Jin 1 , Xiaojie Song 1 , Guoyang Zhang 1 , Shujuan Zhang 1
Affiliation
|
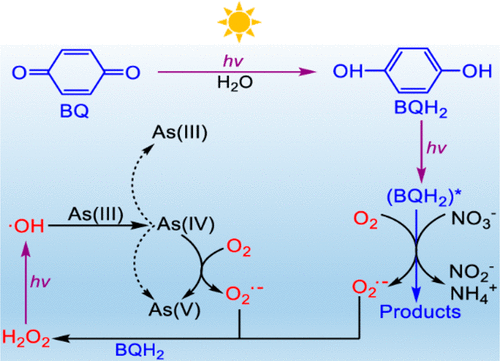
Whether superoxide radical anion (O2•–) was a key reactive species in the oxidation of arsenite (As(III)) in photochemical processes has long been a controversial issue. With hydroquinone (BQH2) and 1,4-benzoquinone (BQ) as redox mediators, the photochemical oxidation of As(III) and reduction of nitrate (NO3–) was carefully investigated. O2•–, singlet oxygen (1O2), H2O2, and semiquinone radical (BQH•) were all possible reactive species in the irradiated system. However, since the formation of As(IV) is a necessary step in the oxidation of As(III), taking the standard reduction potentials into account, the reactions between the above species and As(III) were thermodynamically unfavorable. On the basis of radical scavenging experiments, hydroxyl radical (•OH) was proved as the key species that led to the oxidation of As(III) in the UV/BQH2 system. It should be noted that the •OH radicals were generated from the photolysis of H2O2, which came from the disproportionation of O2•– and the reaction of O2•– with BQH2. Both the photoejected eaq– from 1(BQH2)* and the direct electron transfer with 3(BQH2)* contributed to the reduction of NO3– in the UV/BQH2 process. No synergistic effect was observed in the redox conversion of As(III) and NO3–, further demonstrating that the role of BQH• was negligible in the studied systems. The results here are helpful for a better understanding of the photochemical behaviors of quinones in the aquatic environment.
中文翻译:

UV /醌体系中亚砷酸盐和硝酸盐的氧化还原转化
在光化学过程中,超氧自由基阴离子(O 2 •–)是否是亚砷酸(As(III))氧化中的关键反应物种,一直是一个有争议的问题。用氢醌(BQH 2)和1,4-苯醌(BQ)作为氧化还原介体,如(III)的光化学氧化和还原的硝酸盐(NO 3 - )小心地调查。O 2 •–,单线态氧(1 O 2),H 2 O 2和半醌自由基(BQH •)是辐照系统中所有可能的反应物种。然而,由于As(IV)的形成是As(III)的氧化中的必要步骤,因此考虑到标准还原电位,上述物质与As(III)之间的反应在热力学上是不利的。在自由基清除实验的基础上,羟基自由基(• OH)被证明是导致UV / BQH 2系统中As(III)氧化的关键物质。应该指出的是,• OH自由基是由H 2 O 2的光解产生的,这是由O 2 •–的歧化以及O 2 •–与BQH 2的反应引起的。。两者photoejected ë水溶液-从1(BQH 2)*,并与直接电子转移3(BQH 2)*促进了NO的还原3 -在UV / BQH 2处理。在作为(III)和NO的氧化还原转换没有观察到协同效应3 - ,进一步证明,认为BQH的作用•是在所研究的系统可以忽略不计。此处的结果有助于更好地了解醌在水生环境中的光化学行为。
更新日期:2018-08-14
中文翻译:

UV /醌体系中亚砷酸盐和硝酸盐的氧化还原转化
在光化学过程中,超氧自由基阴离子(O 2 •–)是否是亚砷酸(As(III))氧化中的关键反应物种,一直是一个有争议的问题。用氢醌(BQH 2)和1,4-苯醌(BQ)作为氧化还原介体,如(III)的光化学氧化和还原的硝酸盐(NO 3 - )小心地调查。O 2 •–,单线态氧(1 O 2),H 2 O 2和半醌自由基(BQH •)是辐照系统中所有可能的反应物种。然而,由于As(IV)的形成是As(III)的氧化中的必要步骤,因此考虑到标准还原电位,上述物质与As(III)之间的反应在热力学上是不利的。在自由基清除实验的基础上,羟基自由基(• OH)被证明是导致UV / BQH 2系统中As(III)氧化的关键物质。应该指出的是,• OH自由基是由H 2 O 2的光解产生的,这是由O 2 •–的歧化以及O 2 •–与BQH 2的反应引起的。。两者photoejected ë水溶液-从1(BQH 2)*,并与直接电子转移3(BQH 2)*促进了NO的还原3 -在UV / BQH 2处理。在作为(III)和NO的氧化还原转换没有观察到协同效应3 - ,进一步证明,认为BQH的作用•是在所研究的系统可以忽略不计。此处的结果有助于更好地了解醌在水生环境中的光化学行为。































 京公网安备 11010802027423号
京公网安备 11010802027423号