Journal of Controlled Release ( IF 10.5 ) Pub Date : 2018-08-01 , DOI: 10.1016/j.jconrel.2018.07.048 Xiaobing Wang , Fei Yan , Xiufang Liu , Pan Wang , Shuai Shao , Yue Sun , Zonghai Sheng , Quanhong Liu , Jonathan F. Lovell , Hairong Zheng
|
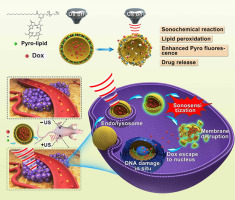
Small molecules that interfere with nucleic acid are widely used in chemotherapy, however, improved delivery approaches are required to improve anti-tumor outcomes. Here, we present the development of an ultrasound-activatable porphyrin-phospholipid-liposome (pp-lipo) that responds to low intensity focused ultrasound (LIFU) for sonodynamic therapy (SDT). The pp-lipo is constructed by incorporating a small proportion of porphyrin (pyropheophorbide) conjugated lipid into a liposome formulation. This enables sonosensitization-induced lipid oxidation and efficient disruption of liposomes to release loaded doxorubicin (Dox). This results in increased Dox nuclear subcellular location and cytotoxicity in cancer cells in vitro upon pp-lipo exposure to LIFU. Following intravenous administration, LIFU enhanced deposition of Dox within tumor tissue, suppressed tumor growth, and also increased porphyrin near infrared tumor fluorescence. Thus, pp-lipo is a versatile carrier that can be extended to many ultrasound-controllable drug delivery applications.
中文翻译:

使用可声活化的脂质体与膜包埋的卟啉增强药物递送
干扰核酸的小分子已广泛用于化学疗法,但是,需要改进的递送方法以改善抗肿瘤效果。在这里,我们介绍了超声激活的卟啉-磷脂-脂质体(pp-lipo)的发展,该反应对低强度聚焦超声(LIFU)进行了声动力疗法(SDT)的响应。pp-脂质是通过将少量卟啉(焦脱镁叶绿素)共轭脂质掺入脂质体制剂中而构建的。这使得声致敏诱导的脂质氧化和脂质体的有效破坏释放出负载的阿霉素(Dox)。这导致体外癌细胞中的Dox核亚细胞定位和细胞毒性增加pp-脂质暴露于LIFU后。静脉内给药后,LIFU增强了肿瘤组织内Dox的沉积,抑制了肿瘤的生长,并且还增加了卟啉近红外肿瘤荧光。因此,pp-lipo是一种通用的载体,可以扩展到许多超声可控的药物递送应用中。































 京公网安备 11010802027423号
京公网安备 11010802027423号