当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
3-Formylpyrazolo[1,5-a]pyrimidines as Key Intermediates for the Preparation of Functional Fluorophores
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-07-27 00:00:00 , DOI: 10.1021/acs.joc.8b01571 Juan-Carlos Castillo 1, 2 , Alexis Tigreros 1 , Jaime Portilla 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-07-27 00:00:00 , DOI: 10.1021/acs.joc.8b01571 Juan-Carlos Castillo 1, 2 , Alexis Tigreros 1 , Jaime Portilla 1
Affiliation
|
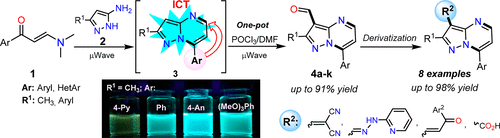
A one-pot route for the regioselective synthesis of 3-formylpyrazolo[1,5-a]pyrimidines 4a–k in good yields through a microwave-assisted process is provided. The synthesis proceeds via a cyclocondensation reaction between β-enaminones 1 with NH-3-aminopyrazoles 2, followed by formylation with an iminium salt moiety (Vilsmeyer–Haack reagent). These N-heteroaryl aldehydes 4 were successfully used as strategic intermediates for the preparation of novel functional fluorophores with yields up to 98%. The structures of the products obtained and regioselectivity of the reactions were determined on the basis of NMR measurements and X-ray diffraction analysis. Since pyrazolo[1,5-a]pyrimidines (PPs) 3 have shown an important fluorescence, photophysical properties of four 2-methylderivatives substituted at position 7 with different acceptor (A) or donor (D) groups were investigated. The compounds evaluated exhibited large Stokes shift in different solvents, but only the substituted p-methoxyphenyl (4-An) showed a strong fluorescence intensity with quantum yields up to 44% due to its greater ICT. Therefore, hybrid systems based on pyrazolo[1,5-a]pyrimidines could be used as fluorescent probes to detect biologically or environmentally relevant species.
中文翻译:

3-甲酰基吡唑并[1,5-a]嘧啶作为制备功能性荧光团的关键中间体
提供了一种通过微波辅助过程以良好产率区域选择性合成 3-甲酰基吡唑并[1,5- a ]嘧啶4a – k的一锅法路线。该合成通过 β-烯胺酮1与NH -3-氨基吡唑2之间的环缩合反应进行,然后用亚胺盐部分(Vilsmeyer-Haack 试剂)进行甲酰化。这些N-杂芳基醛4已成功用作制备新型功能性荧光团的战略中间体,收率高达98%。基于NMR测量和X射线衍射分析确定了所得产物的结构和反应的区域选择性。由于吡唑并[1,5- a ]嘧啶(PP) 3显示出重要的荧光,因此研究了在7位上被不同受体(A)或供体(D)基团取代的四种2-甲基衍生物的光物理性质。所评估的化合物在不同溶剂中表现出较大的斯托克斯位移,但只有取代的对甲氧基苯基(4-An)由于其较高的ICT而表现出很强的荧光强度,量子产率高达44%。因此,基于吡唑并[1,5- a ]嘧啶的混合系统可用作荧光探针来检测生物或环境相关物种。
更新日期:2018-07-27
中文翻译:

3-甲酰基吡唑并[1,5-a]嘧啶作为制备功能性荧光团的关键中间体
提供了一种通过微波辅助过程以良好产率区域选择性合成 3-甲酰基吡唑并[1,5- a ]嘧啶4a – k的一锅法路线。该合成通过 β-烯胺酮1与NH -3-氨基吡唑2之间的环缩合反应进行,然后用亚胺盐部分(Vilsmeyer-Haack 试剂)进行甲酰化。这些N-杂芳基醛4已成功用作制备新型功能性荧光团的战略中间体,收率高达98%。基于NMR测量和X射线衍射分析确定了所得产物的结构和反应的区域选择性。由于吡唑并[1,5- a ]嘧啶(PP) 3显示出重要的荧光,因此研究了在7位上被不同受体(A)或供体(D)基团取代的四种2-甲基衍生物的光物理性质。所评估的化合物在不同溶剂中表现出较大的斯托克斯位移,但只有取代的对甲氧基苯基(4-An)由于其较高的ICT而表现出很强的荧光强度,量子产率高达44%。因此,基于吡唑并[1,5- a ]嘧啶的混合系统可用作荧光探针来检测生物或环境相关物种。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号