Water Research ( IF 11.4 ) Pub Date : 2018-07-27 , DOI: 10.1016/j.watres.2018.07.061 Xianshi Wang , Yulei Liu , Zhuangsong Huang , Lu Wang , Yicheng Wang , Yanting Li , Juan Li , Jingyao Qi , Jun Ma
|
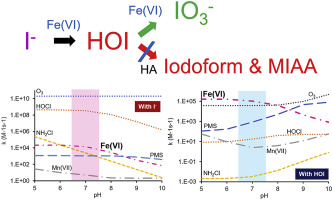
Toxic and odorous iodinated disinfection byproducts (I-DBPs) could form in the chemical oxidation of iodine-containing water. A critical step for controlling the hazardous I-DBPs is to convert the iodine species into stable and harmless iodate (IO3−) while inhibiting the accumulation of highly reactive hypoiodous acid (HOI). Herein, the oxidation of I− and HOI with ferrate was investigated, and the formation profile of HOI during the oxidation of I− was determined based on 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) coloring method through a stopped-flow spectrophotometer. The second-order rate constants (kapp) of ferrate with HOI decreased from 1.6 × 105 M−1s−1 to 8.3 × 102 M−1s−1 as the solution pH varied from 5.3 to 10.3, which were 7.5, 7.2 and 13.8 times higher than that of ferrate with I− at pH 6.0, 7.0 and 8.0, respectively. Compared with other oxidants such as ozone, hypochlorous acid, chloramine and potassium permanganate, ferrate would swiftly oxidize HOI formed in the I− oxidation process. For the ferrate oxidation of I-containing water, HOI was swiftly oxidized to IO3− from pH 5.0 to 9.0. Phosphate buffer promoted the oxidation of I− while inhibited the oxidation of HOI with ferrate. When 5 mgC/L of humic acids (HA) existed in the solution, no formation of iodoform and monoiodoacetic acid (MIAA) was observed in the oxidation of iodide (20 μM) with ferrate (from 10 μM to 80 μM). These results have important implications for the control of I-DBPs in water treatment.
中文翻译:

碘化物的快速氧化和次碘酸与高铁酸盐和高铁酸盐/ I没有形成碘仿和碘乙酸的- / HA系统
含碘水的化学氧化过程中可能会形成有毒和有臭味的碘化消毒副产物(I-DBP)。用于控制有害I-DBPs的一个关键步骤是将碘物质转变成稳定和无害碘酸盐(IO 3 - ),而抑制反应性高次碘酸(HOI)的积累。这里,I的氧化-和HOI与高铁酸盐进行了研究,并且I的氧化期间HOI的形成轮廓-基于2,2'-连氮-双(3-乙基苯并噻唑-6-磺酸)(ABTS)测定通过停止流式分光光度计进行着色。高铁酸盐与HOI的二阶速率常数(k app)从1.6×10 5 M -1降低小号-1至8.3×10 2 中号-1小号-1作为溶液的pH值变化范围为5.3至10.3,这是比用我的高铁酸盐高7.5,7.2和13.8倍-分别为6.0,7.0和8.0,在pH值。与其它氧化剂如臭氧,次氯酸,氯胺相比和高锰酸钾,高铁酸盐会迅速形成在I氧化HOI -氧化过程。对于包含I-水的高铁酸盐氧化,HOI被迅速氧化,IO 3 -从pH为5.0至9.0。磷酸盐缓冲液促进了我的氧化-同时抑制了高铁酸盐对HOI的氧化作用。当溶液中存在5 mgC / L的腐殖酸(HA)时,在高铁酸盐(10μM至80μM)的碘化物(20μM)氧化过程中,未观察到碘仿和单碘乙酸(MIAA)的形成。这些结果对水处理中I-DBPs的控制具有重要意义。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号