当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design, synthesis, and evaluation of 4,5,6,7-tetrahydrobenzo[d]thiazole-based novel dual kinase inhibitors of CK2 and GSK3β†
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2018-07-26 00:00:00 , DOI: 10.1039/c8md00321a Triveni R Pardhi 1 , Manishkumar S Patel 2 , V Sudarsanam 3 , Kamala K Vasu 1, 3
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2018-07-26 00:00:00 , DOI: 10.1039/c8md00321a Triveni R Pardhi 1 , Manishkumar S Patel 2 , V Sudarsanam 3 , Kamala K Vasu 1, 3
Affiliation
|
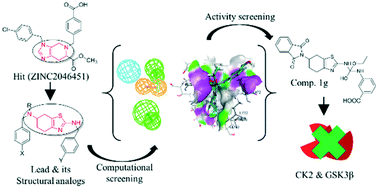
Casein kinase 2 (CK2) and glycogen synthase kinase-3beta (GSK3β) are responsible for the phosphorylation of a tumor suppressor protein (PTEN) in a cooperative manner which causes its deactivation. Thus, it is essential to inhibit both kinases simultaneously to prevent PTEN deactivation more efficiently. In this study, we have designed a novel lead from Hit15 which was identified in silico as a dual kinase inhibitor against CK2 and GSK3β through our previous study. The dataset of structural analogs of the lead was designed and confirmed by pharmacophore mapping and molecular docking. The screened analogs were considered further and a series of “tetrahydrobenzo[d]thiazoles” were synthesized. Compound 1g has shown highest dual kinase inhibitory activity at a concentration of 1.9 μM against CK2 and 0.67 μM against GSK3β. Our results suggest that the presence of a carboxyl group at the meta position of the phenyl ring plays a vital role in dual kinase inhibition.
中文翻译:

基于 4,5,6,7-四氢苯并[d]噻唑的新型 CK2 和 GSK3β 双激酶抑制剂的设计、合成和评估
酪蛋白激酶 2 (CK2) 和糖原合酶激酶-3β (GSK3β) 以协同方式负责肿瘤抑制蛋白 (PTEN) 的磷酸化,从而导致其失活。因此,必须同时抑制两种激酶以更有效地防止 PTEN 失活。在这项研究中,我们设计了一种来自 Hit15 的新型先导物,通过我们之前的研究,该先导物在计算机上被鉴定为针对 CK2 和 GSK3β 的双重激酶抑制剂。通过药效团作图和分子对接设计并确认了先导结构类似物的数据集。进一步考虑筛选的类似物,合成了一系列“四氢苯并[ d ]噻唑”。复方1g在浓度为 1.9 μM 对 CK2 和 0.67 μM 对 GSK3β 时显示出最高的双激酶抑制活性。我们的结果表明,在苯环的间位存在羧基在双重激酶抑制中起着至关重要的作用。
更新日期:2018-07-26
中文翻译:

基于 4,5,6,7-四氢苯并[d]噻唑的新型 CK2 和 GSK3β 双激酶抑制剂的设计、合成和评估
酪蛋白激酶 2 (CK2) 和糖原合酶激酶-3β (GSK3β) 以协同方式负责肿瘤抑制蛋白 (PTEN) 的磷酸化,从而导致其失活。因此,必须同时抑制两种激酶以更有效地防止 PTEN 失活。在这项研究中,我们设计了一种来自 Hit15 的新型先导物,通过我们之前的研究,该先导物在计算机上被鉴定为针对 CK2 和 GSK3β 的双重激酶抑制剂。通过药效团作图和分子对接设计并确认了先导结构类似物的数据集。进一步考虑筛选的类似物,合成了一系列“四氢苯并[ d ]噻唑”。复方1g在浓度为 1.9 μM 对 CK2 和 0.67 μM 对 GSK3β 时显示出最高的双激酶抑制活性。我们的结果表明,在苯环的间位存在羧基在双重激酶抑制中起着至关重要的作用。








































 京公网安备 11010802027423号
京公网安备 11010802027423号