Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Role of Ninth Type-III Domain of Fibronectin in the Mediation of Cell-Binding Domain Adsorption on Surfaces with Different Chemistries
Langmuir ( IF 3.7 ) Pub Date : 2018-07-25 00:00:00 , DOI: 10.1021/acs.langmuir.8b01937 Tianjie Li 1 , Lijing Hao 1 , Jiangyu Li 2 , Chang Du 1 , Yingjun Wang 1
Langmuir ( IF 3.7 ) Pub Date : 2018-07-25 00:00:00 , DOI: 10.1021/acs.langmuir.8b01937 Tianjie Li 1 , Lijing Hao 1 , Jiangyu Li 2 , Chang Du 1 , Yingjun Wang 1
Affiliation
|
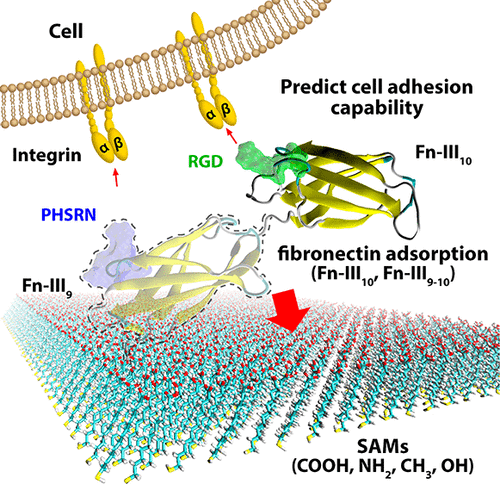
The orientation and conformation of adhesive proteins after adsorption play a central role in cell-binding bioactivity. Fibronectin (Fn) holds two peptide sequences that favor cell adhesion: the Arg–Gly–Asp (RGD) loop on the tenth type-III domain (Fn-III10) and the Pro–His–Ser–Arg–Asn (PHSRN) synergy site on the ninth type-III domain (Fn-III9). Herein, adsorption of Fn fragments (Fn-III10 and Fn-III9–10) on self-assembled monolayers (SAMs) carrying various functional groups (−COOH, −NH2, −CH3, and −OH) was investigated by the Monte Carlo method and molecular dynamics simulation in order to understand its mediation effect on cell adhesion. The results demonstrated that Fn-III9 could enhance the stiffness of the Fn molecule and further fix the adsorption orientation. The RGD site of the Fn fragment appeared to be deactivated on hydrophobic surfaces (CH3-SAM) because of the binding of adjacent nonpolar residues on surfaces, whereas charged surfaces (COOH-SAM and NH2-SAM) and hydrophilic surfaces (OH-SAM) were conducive to the formation of cell-binding-favorable orientation for Fn fragments. The cell adhesion capability of Fn fragments was highly improved on positively charged surfaces (NH2-SAM) and hydrophilic surfaces because of the advantageous steric structure and orientation of both RGD and PHSRN sites. This work provides an insight into the interplay at the atomic scale between protein adsorption and surface chemistry for designing biologically responsive substrate surfaces.
中文翻译:

纤连蛋白的第III类III结构域在不同化学物质表面介导的细胞结合结构域吸附中的作用
吸附后粘附蛋白的方向和构象在细胞结合生物活性中起着核心作用。纤连蛋白(Fn)具有两个有利于细胞粘附的肽序列:第十个III型结构域(Fn-III 10)上的Arg-Gly-Asp(RGD)环和Pro-His-Ser-Arg-Asn(PHSRN)在第III型III结构域(Fn-III 9)上的协同位点。在本文中,通过以下方法研究了Fn片段(Fn-III 10和Fn-III 9-10)在带有各种官能团(-COOH,-NH 2,-CH 3和-OH)的自组装单分子膜(SAMs)上的吸附。为了了解其对细胞黏附的介导作用,使用了蒙特卡洛方法和分子动力学模拟。结果表明,Fn-III9可以增强Fn分子的刚度并进一步固定吸附方向。Fn片段的RGD位点似乎在疏水表面(CH 3 -SAM)上失活,因为表面上相邻的非极性残基结合,而带电表面(COOH-SAM和NH 2 -SAM)和亲水表面(OH- SAM)有利于Fn片段的细胞结合有利方向的形成。Fn片段在带正电的表面(NH 2-SAM)和亲水性表面,因为RGD和PHSRN位置均具有有利的空间结构和方向。这项工作提供了一种洞察力,以了解蛋白质吸附和表面化学之间在原子尺度上的相互作用,以设计具有生物响应性的底物表面。
更新日期:2018-07-25
中文翻译:

纤连蛋白的第III类III结构域在不同化学物质表面介导的细胞结合结构域吸附中的作用
吸附后粘附蛋白的方向和构象在细胞结合生物活性中起着核心作用。纤连蛋白(Fn)具有两个有利于细胞粘附的肽序列:第十个III型结构域(Fn-III 10)上的Arg-Gly-Asp(RGD)环和Pro-His-Ser-Arg-Asn(PHSRN)在第III型III结构域(Fn-III 9)上的协同位点。在本文中,通过以下方法研究了Fn片段(Fn-III 10和Fn-III 9-10)在带有各种官能团(-COOH,-NH 2,-CH 3和-OH)的自组装单分子膜(SAMs)上的吸附。为了了解其对细胞黏附的介导作用,使用了蒙特卡洛方法和分子动力学模拟。结果表明,Fn-III9可以增强Fn分子的刚度并进一步固定吸附方向。Fn片段的RGD位点似乎在疏水表面(CH 3 -SAM)上失活,因为表面上相邻的非极性残基结合,而带电表面(COOH-SAM和NH 2 -SAM)和亲水表面(OH- SAM)有利于Fn片段的细胞结合有利方向的形成。Fn片段在带正电的表面(NH 2-SAM)和亲水性表面,因为RGD和PHSRN位置均具有有利的空间结构和方向。这项工作提供了一种洞察力,以了解蛋白质吸附和表面化学之间在原子尺度上的相互作用,以设计具有生物响应性的底物表面。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号