当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the Atmospheric Oxidation of HFE-7500 (C3F7CF(OC2H5)CF(CF3)2) Initiated by Cl Atom and NO3 Radical from Theory
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-07-25 00:00:00 , DOI: 10.1021/acs.jpca.8b04225
Pradeep Kumar Rao 1 , Ramesh Ch. Deka 2 , Nand Kishor Gour 2 , Shridhar P. Gejji 3
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-07-25 00:00:00 , DOI: 10.1021/acs.jpca.8b04225
Pradeep Kumar Rao 1 , Ramesh Ch. Deka 2 , Nand Kishor Gour 2 , Shridhar P. Gejji 3
Affiliation
|
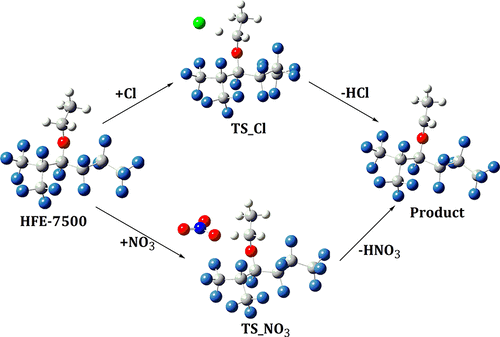
Kinetics and mechanistic pathways for atmospheric oxidation of HFE-7500 (n-C3F7CF(OCH2CH3)CF(CF3)2) initiated by Cl atom and NO3 radical have been studied using density functional theory. Oxidative degradation pathways facilitated by H-abstraction from the −OCH2 or −CH3 groups in HFE-7500 have been considered. It has been shown that H-abstraction from the α-site (−OCH2) is favored over other reaction pathways. The rate constants were computed employing transition-state theory and canonical variation transition-state theory incorporating small curvature tunnelling correction, over the temperature range of 250–450 K at atmospheric pressure. Calculated rate constants at 298 K and 1 atm compare well with earlier experiments. Temperature dependence of the rate constants and branching ratios for these pathways contributing to overall reaction are described. It has been shown that the rate constants over the studied temperature range was found to fit well to the modified Arrhenius equation (in cm3 molecule–1 s–1) kCl = 1.10 × 10–14 T0.04 exp(−69.87 ± 1.41/T) and kNO3 = 7.66 × 10–26T3.30 exp(596.40 ± 1.22/T). Standard enthalpies of formation for the reactant (C3F7CF(OCH2CH3)CF(CF3)2) and the products [C3F7CF(OC•HCH3)CF(CF3)2 and C3F7CF(OCH2C•H2)CF(CF3)2] during H-abstraction are derived using the isodesmic approach. Atmospheric implications of the titled molecule are presented.
中文翻译:

从理论上理解Cl原子和NO 3引发的HFE-7500(C 3 F 7 CF(OC 2 H 5)CF(CF 3)2)的大气氧化
利用密度泛函理论研究了由Cl原子和NO 3自由基引发的HFE-7500(n -C 3 F 7 CF(OCH 2 CH 3)CF(CF 3)2)大气氧化的动力学和机理。已经考虑了HFE-7500中从-OCH 2或-CH 3基团中进行H抽提而促进的氧化降解途径。研究表明,从α位(-OCH 2)优于其他反应途径。在大气压下,在250–450 K的温度范围内,采用过渡态理论和典范变化过渡态理论并结合了小曲率隧穿校正来计算速率常数。在298 K和1 atm处计算出的速率常数与早期实验相比较。描述了这些路径的速率常数和分支比对温度的依赖性,这些路径有助于整体反应。结果表明,在研究的温度范围内的速率常数与修正的Arrhenius方程非常吻合(以cm 3分子–1 s –1表示)k Cl = 1.10×10 –14 T 0.04exp(-69.87±1.41 / T)和k NO 3 = 7.66×10 –26 T 3.30 exp(596.40±1.22 / T)。反应物(C 3 F 7 CF(OCH 2 CH 3)CF(CF 3)2)和产物[C 3 F 7 CF(OC • HCH 3)CF(CF 3)2和C 3的标准形成焓。F 7 CF(OCH 2 C • H 2)CF(CF 3)2使用等渗方法导出H提取期间的。介绍了标题分子的大气含义。
更新日期:2018-07-25
中文翻译:

从理论上理解Cl原子和NO 3引发的HFE-7500(C 3 F 7 CF(OC 2 H 5)CF(CF 3)2)的大气氧化
利用密度泛函理论研究了由Cl原子和NO 3自由基引发的HFE-7500(n -C 3 F 7 CF(OCH 2 CH 3)CF(CF 3)2)大气氧化的动力学和机理。已经考虑了HFE-7500中从-OCH 2或-CH 3基团中进行H抽提而促进的氧化降解途径。研究表明,从α位(-OCH 2)优于其他反应途径。在大气压下,在250–450 K的温度范围内,采用过渡态理论和典范变化过渡态理论并结合了小曲率隧穿校正来计算速率常数。在298 K和1 atm处计算出的速率常数与早期实验相比较。描述了这些路径的速率常数和分支比对温度的依赖性,这些路径有助于整体反应。结果表明,在研究的温度范围内的速率常数与修正的Arrhenius方程非常吻合(以cm 3分子–1 s –1表示)k Cl = 1.10×10 –14 T 0.04exp(-69.87±1.41 / T)和k NO 3 = 7.66×10 –26 T 3.30 exp(596.40±1.22 / T)。反应物(C 3 F 7 CF(OCH 2 CH 3)CF(CF 3)2)和产物[C 3 F 7 CF(OC • HCH 3)CF(CF 3)2和C 3的标准形成焓。F 7 CF(OCH 2 C • H 2)CF(CF 3)2使用等渗方法导出H提取期间的。介绍了标题分子的大气含义。







































 京公网安备 11010802027423号
京公网安备 11010802027423号