当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functional Poly(ester–amide)s with Tertiary Ester Linkages via the Passerini Multicomponent Polymerization of a Dicarboxylic Acid and a Diisocyanide with Different Electron-Deficient Ketones
Macromolecules ( IF 5.1 ) Pub Date : 2018-07-25 00:00:00 , DOI: 10.1021/acs.macromol.8b01168
Jian Zhang 1 , Yu-Huan Wu 1 , Jia-Chen Wang 1 , Fu-Sheng Du 1 , Zi-Chen Li 1
Macromolecules ( IF 5.1 ) Pub Date : 2018-07-25 00:00:00 , DOI: 10.1021/acs.macromol.8b01168
Jian Zhang 1 , Yu-Huan Wu 1 , Jia-Chen Wang 1 , Fu-Sheng Du 1 , Zi-Chen Li 1
Affiliation
|
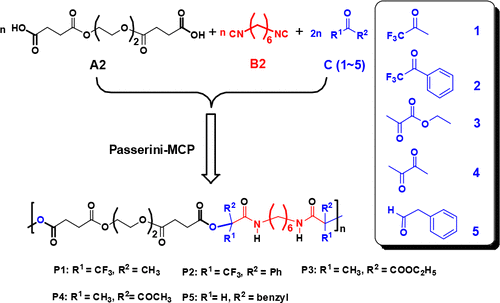
We describe a straightforward synthetic strategy for a new family of functional poly(ester–amide)s (PEAs) with tertiary ester linkages via the Passerini multicomponent polymerization (Passerini-MCP) of a diacid (A2), 1,6-diisocyanohexane (B2), and four kinds of electron-deficient ketones. First, Passerini three-component reactions (Passerni-3CR) of hexanoic acid and tert-butyl isocyanide with nine kinds of ketones were investigated to evaluate the reactivities of these ketones toward Passerni-3CR. Four electron-deficient ketones, 1,1,1-trifluoroacetone (1), 1,1,1-trifluoroacetophenone (2), ethyl pyruvate (3), and 2,3-butanedione (4), were found to be excellent oxo-compounds for this Passerini-3CR. Then, the Passerini-MCP of A2 and B2 with 1, 2, 3, and 4 in CH2Cl2 was performed to generate polymers P1–P4 with tertiary ester linkages and different electron-withdrawing substituents. Polymer P5 was also prepared by the Passerini-MCP of A2 and B2 with phenylacetaldehyde (5) for comparison. Size exclusion chromatography (SEC) verified the high molecular weights (Mn up to 30.6 kDa) of these polymers. The structures of these polymers were confirmed by 1H NMR, 13C NMR, 19F NMR, and matrix-assisted laser desorption ionization mass spectroscopy. Thermal gravimetric analysis (TGA) and differential scanning calorimetry (DSC) revealed that P1–P4 are less stable than polymer P5, and they are amorphous with variable glass transition temperatures (Tg) depending on the side groups. Hydrolytic degradation of P1, P4, and P5 in a mixture of acetonitrile and phosphate buffer (different pH) was investigated, and at the same pH, the degradation follows the order of P4 > P1 > P5. The static water contact angles of thin films formed by these polymers decreased from 96° (P1) to 78° (P2), 50° (P3), and finally to 32° (P4). Tensile properties of polymer P3 and P4 were characterized by dynamic mechanical analysis (DMA). P4 displayed a high tensile stress of 25 MPa with the elongation up to 200%.
中文翻译:

通过二羧酸和二异氰酸酯与不同电子不足的酮的Passerini多组分聚合反应,具有叔酯键的功能性聚(酯-酰胺)
我们描述了一种简单的合成策略为官能化的聚(酯-酰胺)(豌豆)经由帕瑟里尼多组分聚合二酸(帕瑟里尼-MCP)(叔酯键的一个新的家庭A2),1,6- diisocyanohexane(B2)和四种电子不足的酮。首先,研究了己酸和叔丁基异氰化物与9种酮的Passerini三组分反应(Passerni-3CR),以评估这些酮对Passerni-3CR的反应性。四个缺电子的酮:1,1,1-三氟丙酮(1),1,1,1-三氟苯乙酮(2),丙酮酸乙酯(3)和2,3-丁二酮(4),被认为是该Passerini-3CR的优良含氧化合物。然后,的帕瑟里尼-MCP A2和B2与1,2,3,和4在CH 2氯2被执行,以生成的聚合物P1 - P4与叔酯键和不同吸电子取代基。还通过A2和B2的Passerini-MCP与苯乙醛(5)制备了聚合物P5,以作比较。尺寸排阻色谱法(SEC)验证了高分子量(M n高达30.6 kDa的这些聚合物。这些聚合物的结构通过1 H NMR,13 C NMR,19 F NMR和基质辅助激光解吸电离质谱法确认。热重分析(TGA)和差示扫描量热法(DSC)显示,P1 - P4的稳定性低于聚合物P5,并且它们是非晶态的,取决于侧基的不同,玻璃化转变温度(T g)也不同。P1,P4和P5的水解降解在乙腈和磷酸盐缓冲液(不同的pH值)的混合物中进行了研究,在相同的pH值下,降解遵循P4 > P1 > P5的顺序。由这些聚合物形成的薄膜的静态水接触角从96°(P1)降至78°(P2),50°(P3),最后降至32°(P4)。通过动态力学分析(DMA)表征聚合物P3和P4的拉伸性能。P4表现出25 MPa的高拉伸应力,伸长率高达200%。
更新日期:2018-07-25
中文翻译:

通过二羧酸和二异氰酸酯与不同电子不足的酮的Passerini多组分聚合反应,具有叔酯键的功能性聚(酯-酰胺)
我们描述了一种简单的合成策略为官能化的聚(酯-酰胺)(豌豆)经由帕瑟里尼多组分聚合二酸(帕瑟里尼-MCP)(叔酯键的一个新的家庭A2),1,6- diisocyanohexane(B2)和四种电子不足的酮。首先,研究了己酸和叔丁基异氰化物与9种酮的Passerini三组分反应(Passerni-3CR),以评估这些酮对Passerni-3CR的反应性。四个缺电子的酮:1,1,1-三氟丙酮(1),1,1,1-三氟苯乙酮(2),丙酮酸乙酯(3)和2,3-丁二酮(4),被认为是该Passerini-3CR的优良含氧化合物。然后,的帕瑟里尼-MCP A2和B2与1,2,3,和4在CH 2氯2被执行,以生成的聚合物P1 - P4与叔酯键和不同吸电子取代基。还通过A2和B2的Passerini-MCP与苯乙醛(5)制备了聚合物P5,以作比较。尺寸排阻色谱法(SEC)验证了高分子量(M n高达30.6 kDa的这些聚合物。这些聚合物的结构通过1 H NMR,13 C NMR,19 F NMR和基质辅助激光解吸电离质谱法确认。热重分析(TGA)和差示扫描量热法(DSC)显示,P1 - P4的稳定性低于聚合物P5,并且它们是非晶态的,取决于侧基的不同,玻璃化转变温度(T g)也不同。P1,P4和P5的水解降解在乙腈和磷酸盐缓冲液(不同的pH值)的混合物中进行了研究,在相同的pH值下,降解遵循P4 > P1 > P5的顺序。由这些聚合物形成的薄膜的静态水接触角从96°(P1)降至78°(P2),50°(P3),最后降至32°(P4)。通过动态力学分析(DMA)表征聚合物P3和P4的拉伸性能。P4表现出25 MPa的高拉伸应力,伸长率高达200%。































 京公网安备 11010802027423号
京公网安备 11010802027423号