当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrocatalytic Reduction of Nitrate Using Magnéli Phase TiO2 Reactive Electrochemical Membranes Doped with Pd-Based Catalysts
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-08-02 , DOI: 10.1021/acs.est.8b03038
Pralay Gayen 1 , Jason Spataro 1 , Sumant Avasarala 2 , Abdul-Mehdi Ali 3 , José M. Cerrato 2 , Brian P. Chaplin 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-08-02 , DOI: 10.1021/acs.est.8b03038
Pralay Gayen 1 , Jason Spataro 1 , Sumant Avasarala 2 , Abdul-Mehdi Ali 3 , José M. Cerrato 2 , Brian P. Chaplin 1
Affiliation
|
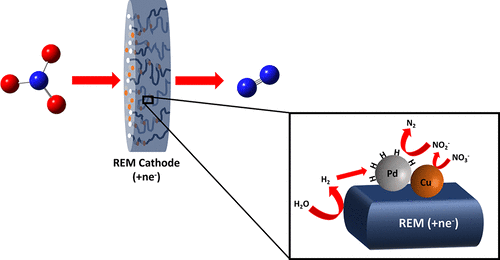
This research focused on synthesis, characterization, and application of point-of-use catalytic reactive electrochemical membranes (REMs) for electrocatalytic NO3– reduction. Deposition of Pd–Cu and Pd–In catalysts to the REMs produced catalytic REMs (i.e., Pd–Cu/REM and Pd–In/REM) that were active for NO3– reduction. Optimal performance was achieved with a Pd–Cu/REM and upstream counter electrode, which reduced NO3– from 1.0 mM to below the EPAs regulatory MCL (700 μM) in a single pass through the REM (residence time ∼2 s), obtaining product selectivity of <2% toward NO2–/NH3. Nitrate reduction was not affected by dissolved oxygen and carbonate species and only slightly decreased in a surface water sample due to Ca2+ and Mg2+ scaling. Energy consumption to treat surface water was 1.1 to 1.3 kWh mol–1 for 1 mM NO3– concentrations, and decreased to 0.19 and 0.12 kWh mol–1 for 10 and 100 mM NaNO3 solutions, respectively. Electrocatalytic reduction kinetics were shown to be an order of magnitude higher than catalytic NO3– reduction kinetics. Conversion of up to 67% of NO3–, with low NO2– (0.7—11 μM) and NH3 formation (<10 μM), and low energy consumption obtained in this study suggest that Pd–Cu/REMs are a promising technology for distributed water treatment.
中文翻译:

Pd基催化剂掺杂的Magnéli相TiO 2反应性电化学膜对硝酸盐的电催化还原
此研究集中在合成,表征,和点使用催化反应的电化学膜(的REM)的应用电催化NO 3 -还原。的Pd-Cu和沉积的Pd-在催化剂到的REM产生催化的REM(即,钯-铜/ REM和Pd-IN / REM),其具有活性为NO 3 -还原。最佳的性能是用钯-铜/ REM和上游侧相对电极,从而减少了NO实现3 -从1.0mM至在单次通过的持久授权书监管MCL(700μM)下面通过REM(停留时间〜2 S),得到对NO 2 – / NH 3的产品选择性<2%。硝酸盐的还原不受溶解氧和碳酸盐种类的影响,由于Ca 2+和Mg 2+的结垢,在地表水样品中硝酸盐的还原仅略有下降。对于1 mM NO 3 –浓度,处理地表水的能耗为1.1至1.3 kWh mol –1,而对于10和100 mM NaNO 3溶液,其能耗分别降至0.19和0.12 kWh mol –1。电催化还原动力学被证明是一个数量级比催化NO更高3 -还原动力学。高达NO的67%的转化率3 - ,具有低NO 2 -(0.7-11μM)和NH 3 这项研究获得的低成因(<10μM)和低能耗表明,Pd-Cu / REMs是用于分布式水处理的有前途的技术。
更新日期:2018-08-02
中文翻译:

Pd基催化剂掺杂的Magnéli相TiO 2反应性电化学膜对硝酸盐的电催化还原
此研究集中在合成,表征,和点使用催化反应的电化学膜(的REM)的应用电催化NO 3 -还原。的Pd-Cu和沉积的Pd-在催化剂到的REM产生催化的REM(即,钯-铜/ REM和Pd-IN / REM),其具有活性为NO 3 -还原。最佳的性能是用钯-铜/ REM和上游侧相对电极,从而减少了NO实现3 -从1.0mM至在单次通过的持久授权书监管MCL(700μM)下面通过REM(停留时间〜2 S),得到对NO 2 – / NH 3的产品选择性<2%。硝酸盐的还原不受溶解氧和碳酸盐种类的影响,由于Ca 2+和Mg 2+的结垢,在地表水样品中硝酸盐的还原仅略有下降。对于1 mM NO 3 –浓度,处理地表水的能耗为1.1至1.3 kWh mol –1,而对于10和100 mM NaNO 3溶液,其能耗分别降至0.19和0.12 kWh mol –1。电催化还原动力学被证明是一个数量级比催化NO更高3 -还原动力学。高达NO的67%的转化率3 - ,具有低NO 2 -(0.7-11μM)和NH 3 这项研究获得的低成因(<10μM)和低能耗表明,Pd-Cu / REMs是用于分布式水处理的有前途的技术。































 京公网安备 11010802027423号
京公网安备 11010802027423号