当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydroxide Solvation and Transport in Anion Exchange Membranes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-01-13 , DOI: 10.1021/jacs.5b11951 Chen Chen 1, 2 , Ying-Lung Steve Tse 1 , Gerrick E. Lindberg 3 , Chris Knight 4 , Gregory A. Voth 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-01-13 , DOI: 10.1021/jacs.5b11951 Chen Chen 1, 2 , Ying-Lung Steve Tse 1 , Gerrick E. Lindberg 3 , Chris Knight 4 , Gregory A. Voth 1
Affiliation
|
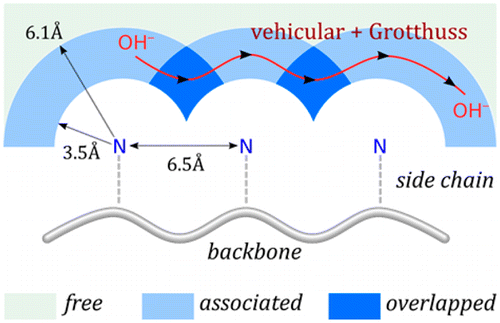
Understanding hydroxide solvation and transport in anion exchange membranes (AEMs) can provide important insight into the design principles of these new membranes. To accurately model hydroxide solvation and transport, we developed a new multiscale reactive molecular dynamics model for hydroxide in aqueous solution, which was then subsequently modified for an AEM material. With this model, we investigated the hydroxide solvation structure and transport mechanism in the membrane. We found that a relatively even separation of the rigid side chains produces a continuous overlapping region for hydroxide transport that is made up of the first hydration shell of the tethered cationic groups. Our results show that hydroxide has a significant preference for this overlapping region, transporting through it and between the AEM side chains with substantial contributions from both vehicular (standard diffusion) and Grotthuss (proton hopping) mechanisms. Comparison of the AEM with common proton exchange membranes (PEMs) showed that the excess charge is less delocalized in the AEM than the PEMs, which is correlated with a higher free energy barrier for proton transfer reactions. The vehicular mechanism also contributes considerably more than the Grotthuss mechanism for hydroxide transport in the AEM, while our previous studies of PEM systems showed a larger contribution from the Grotthuss mechanism than the vehicular mechanism for proton transport. The activation energy barrier for hydroxide diffusion in the AEM is greater than that for proton diffusion in PEMs, implying a more significant enhancement of ion transport in the AEM at elevated temperatures.
中文翻译:

阴离子交换膜中的氢氧化物溶解和运输
了解阴离子交换膜 (AEM) 中的氢氧化物溶剂化和传输可以为了解这些新膜的设计原理提供重要的见解。为了准确模拟氢氧化物的溶剂化和传输,我们开发了一种新的多尺度反应分子动力学模型,用于水溶液中的氢氧化物,随后针对 AEM 材料进行了修改。使用该模型,我们研究了膜中的氢氧化物溶剂化结构和传输机制。我们发现刚性侧链的相对均匀的分离产生了一个连续的重叠区域,用于氢氧化物运输,该区域由束缚的阳离子基团的第一个水合壳组成。我们的结果表明氢氧化物对这个重叠区域有显着的偏好,通过它以及在 AEM 侧链之间传输,具有来自车辆(标准扩散)和 Grotthuss(质子跳跃)机制的大量贡献。AEM 与普通质子交换膜 (PEM) 的比较表明,与 PEM 相比,AEM 中的过量电荷离域更少,这与质子转移反应的更高自由能垒相关。在 AEM 中,车辆机制也比 Grotthuss 机制对氢氧根传输的贡献大得多,而我们之前对 PEM 系统的研究表明,Grotthuss 机制的贡献大于质子传输的车辆机制。AEM 中氢氧化物扩散的活化能垒大于 PEM 中质子扩散的活化能垒,
更新日期:2016-01-13
中文翻译:

阴离子交换膜中的氢氧化物溶解和运输
了解阴离子交换膜 (AEM) 中的氢氧化物溶剂化和传输可以为了解这些新膜的设计原理提供重要的见解。为了准确模拟氢氧化物的溶剂化和传输,我们开发了一种新的多尺度反应分子动力学模型,用于水溶液中的氢氧化物,随后针对 AEM 材料进行了修改。使用该模型,我们研究了膜中的氢氧化物溶剂化结构和传输机制。我们发现刚性侧链的相对均匀的分离产生了一个连续的重叠区域,用于氢氧化物运输,该区域由束缚的阳离子基团的第一个水合壳组成。我们的结果表明氢氧化物对这个重叠区域有显着的偏好,通过它以及在 AEM 侧链之间传输,具有来自车辆(标准扩散)和 Grotthuss(质子跳跃)机制的大量贡献。AEM 与普通质子交换膜 (PEM) 的比较表明,与 PEM 相比,AEM 中的过量电荷离域更少,这与质子转移反应的更高自由能垒相关。在 AEM 中,车辆机制也比 Grotthuss 机制对氢氧根传输的贡献大得多,而我们之前对 PEM 系统的研究表明,Grotthuss 机制的贡献大于质子传输的车辆机制。AEM 中氢氧化物扩散的活化能垒大于 PEM 中质子扩散的活化能垒,






























 京公网安备 11010802027423号
京公网安备 11010802027423号