当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Synthesis of Chiral 1,2-Amino Alcohols and Morpholin-2-ones from Arylglyoxals
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-07-24 00:00:00 , DOI: 10.1021/acs.joc.8b01516
Wyatt C. Powell 1 , Maciej A. Walczak 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-07-24 00:00:00 , DOI: 10.1021/acs.joc.8b01516
Wyatt C. Powell 1 , Maciej A. Walczak 1
Affiliation
|
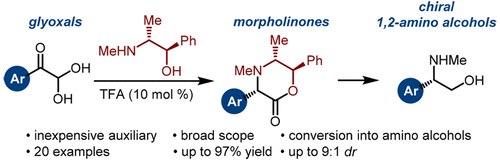
Chiral 1,2-amino alcohols are privileged scaffolds with important applications as drug candidates and chiral ligands. Although various methods for the preparation of this structural motif have been reported, these methods are limited because of the use of precious metals and ligands. Here, we report a practical and high yielding synthesis of chiral 1,2-amino alcohols using arylglyoxals and pseudoephedrine auxiliary. This reaction is catalyzed by a Brønsted acid and provides morpholinone products in high yields and selectivities. The morpholine ring was converted into 1,2-amino alcohols in a two-step protocol.
中文翻译:

由芳基乙二醛不对称合成手性1,2-氨基醇和吗啉-2-酮
手性1,2-氨基醇是特有的支架,在候选药物和手性配体方面具有重要的应用。尽管已经报道了各种制备该结构基序的方法,但是由于使用了贵金属和配体,这些方法受到了限制。在这里,我们报道了使用芳基乙二醛和伪麻黄碱助剂的一种实用且高产的手性1,2-氨基醇的合成方法。该反应由布朗斯台德酸催化,以高收率和选择性提供吗啉酮产物。通过两步操作,将吗啉环转化为1,2-氨基醇。
更新日期:2018-07-24
中文翻译:

由芳基乙二醛不对称合成手性1,2-氨基醇和吗啉-2-酮
手性1,2-氨基醇是特有的支架,在候选药物和手性配体方面具有重要的应用。尽管已经报道了各种制备该结构基序的方法,但是由于使用了贵金属和配体,这些方法受到了限制。在这里,我们报道了使用芳基乙二醛和伪麻黄碱助剂的一种实用且高产的手性1,2-氨基醇的合成方法。该反应由布朗斯台德酸催化,以高收率和选择性提供吗啉酮产物。通过两步操作,将吗啉环转化为1,2-氨基醇。































 京公网安备 11010802027423号
京公网安备 11010802027423号