当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Functionalization of Achmatowicz Rearrangement Products by Reactions with Potassium Organotrifluoroborates under Transition-Metal-Free Conditions
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-07-23 00:00:00 , DOI: 10.1021/acs.joc.8b01643 Silvia Roscales 1 , Víctor Ortega 1 , Aurelio G. Csákÿ 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-07-23 00:00:00 , DOI: 10.1021/acs.joc.8b01643 Silvia Roscales 1 , Víctor Ortega 1 , Aurelio G. Csákÿ 1
Affiliation
|
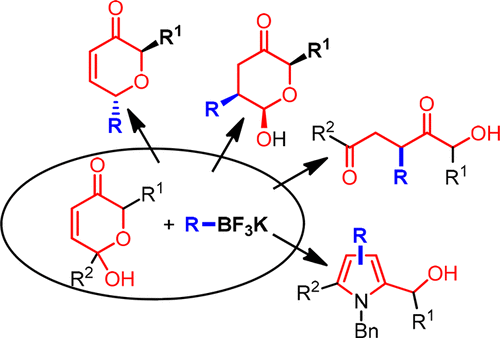
The repertoire of synthetic transformations of the products of the Achmatowicz rearrangement has been expanded by exploring their reactivity with potassium organotrifluoroborates in the absence of transition metals. Depending on the reaction conditions and the substitution pattern of the starting material, the reaction may lead to the stereoselective synthesis of dihydropyranones (2,6-trans), tetrahydropyranones (2,3-cis-2,6-cis) or functionalized 1,4-dicarbonyl compounds. The method has also been adapted for the one-pot synthesis of functionalized pyrroles.
中文翻译:

在无过渡金属的条件下,通过与有机三氟硼酸钾的反应对Achmatowicz重排产物进行选择性功能化
通过在不存在过渡金属的情况下探索其与有机三氟硼酸钾的反应性,扩大了Achmatowicz重排产物的合成转化的范围。根据反应条件和原料的取代方式,反应可能会导致立体选择性合成二氢吡喃酮(2,6-反式),四氢吡喃酮(2,3-顺式-2,6-顺式)或官能化的1, 4-二羰基化合物。该方法还适用于一锅合成官能化吡咯。
更新日期:2018-07-23
中文翻译:

在无过渡金属的条件下,通过与有机三氟硼酸钾的反应对Achmatowicz重排产物进行选择性功能化
通过在不存在过渡金属的情况下探索其与有机三氟硼酸钾的反应性,扩大了Achmatowicz重排产物的合成转化的范围。根据反应条件和原料的取代方式,反应可能会导致立体选择性合成二氢吡喃酮(2,6-反式),四氢吡喃酮(2,3-顺式-2,6-顺式)或官能化的1, 4-二羰基化合物。该方法还适用于一锅合成官能化吡咯。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号