当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereodivergent Rhodium(III)-Catalyzed cis-Cyclopropanation Enabled by Multivariate Optimization
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-07-23 , DOI: 10.1021/jacs.8b04243 Tiffany Piou 1 , Fedor Romanov-Michailidis 1 , Melissa A Ashley 1 , Maria Romanova-Michaelides 2 , Tomislav Rovis 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-07-23 , DOI: 10.1021/jacs.8b04243 Tiffany Piou 1 , Fedor Romanov-Michailidis 1 , Melissa A Ashley 1 , Maria Romanova-Michaelides 2 , Tomislav Rovis 1
Affiliation
|
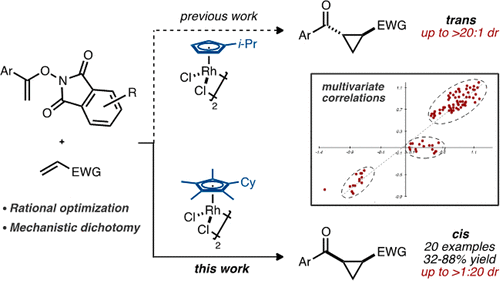
The design of stereodivergent transformations is of great interest to the synthetic community as it allows funneling of a given reaction pathway toward one stereochemical outcome or another by only minor adjustments of the reaction setup. Herein, we present a physical organic approach to invert the sense of induction in diastereoselective cyclopropanation of alkenes with N-enoxyphthalimides through rhodium(III) catalysis. Careful parametrization of catalyst-substrate molecular determinants allowed us to interrogate linear-free energy relationships and establish an intuitive and robust statistical model that correlates an extensive number of data points in high accuracy. Our multivariate correlations-steered mechanistic investigation culminated with a robust and general diastereodivergent cyclopropanation tool where the switch from trans- to cis-diastereoinduction is attributed to a mechanistic dichotomy. Selectivity might be determined by the flexibility of rhodacyclic intermediates derived from ring-opened versus -unopened phthalimides, induced by both their respective ring size and the Sterimol B1 parameter of the CpX ligand on rhodium.
中文翻译:

通过多元优化实现立体发散铑 (III) 催化顺式环丙烷化
立体发散转化的设计引起了合成界的极大兴趣,因为它只需对反应设置进行微小调整,就可以将给定的反应途径集中到一种立体化学结果或另一种立体化学结果。在此,我们提出了一种物理有机方法,通过铑(III)催化反转烯烃与N-烯氧基邻苯二甲酰亚胺的非对映选择性环丙烷化反应中的诱导意义。催化剂-基质分子决定因素的仔细参数化使我们能够询问线性自由能量关系,并建立直观且稳健的统计模型,该模型以高精度关联大量数据点。我们的多变量相关性引导的机械研究最终得到了一个强大且通用的非对映发散环丙烷化工具,其中从反式非对映诱导到顺式非对映诱导的转变归因于机械二分法。选择性可能取决于衍生自开环与未开环邻苯二甲酰亚胺的铑环中间体的灵活性,这是由它们各自的环大小和铑上 CpX 配体的 Sterimol B1 参数引起的。
更新日期:2018-07-23
中文翻译:

通过多元优化实现立体发散铑 (III) 催化顺式环丙烷化
立体发散转化的设计引起了合成界的极大兴趣,因为它只需对反应设置进行微小调整,就可以将给定的反应途径集中到一种立体化学结果或另一种立体化学结果。在此,我们提出了一种物理有机方法,通过铑(III)催化反转烯烃与N-烯氧基邻苯二甲酰亚胺的非对映选择性环丙烷化反应中的诱导意义。催化剂-基质分子决定因素的仔细参数化使我们能够询问线性自由能量关系,并建立直观且稳健的统计模型,该模型以高精度关联大量数据点。我们的多变量相关性引导的机械研究最终得到了一个强大且通用的非对映发散环丙烷化工具,其中从反式非对映诱导到顺式非对映诱导的转变归因于机械二分法。选择性可能取决于衍生自开环与未开环邻苯二甲酰亚胺的铑环中间体的灵活性,这是由它们各自的环大小和铑上 CpX 配体的 Sterimol B1 参数引起的。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号