Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-07-23 , DOI: 10.1016/j.bioorg.2018.07.025 Zhi-Qiang Liu , Huan-Huan Yin , Xiao-Jian Zhang , Rong Zhou , Yan-Mei Wang , Yu-Guo Zheng
|
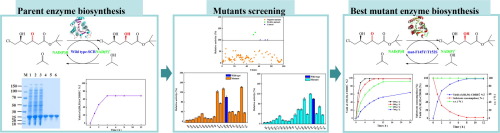
tert-Butyl (3R,5S)-6-chloro-3,5-dihydroxyhexanoate ((3R,5S)-CDHH) is a key chiral intermediate for the side chain synthesis of rosuvastatin. In this study, random mutagenesis, site-saturation mutagenesis and combinatorial mutagenesis methods were applied to improve the activity of a synthesized stereoselective short chain carbonyl reductase (SCR) to prepare (3R,5S)-CDHH. After screened by high-throughput screening method and high-performance liquid chromatography, mut-Phe145Met/Thr152Ser and mut-Phe145Tyr/Thr152Ser, were obtained, and the enzyme activities of mutants were improved by 1.60- and 1.91-fold compared with parent enzyme, respectively. The catalytically efficiencies (kcat/Km) of mut-Phe145Met/Thr152Ser and mut-Phe145Tyr/Thr152Ser exhibited 5.11- and 8.07-fold improvements in initial activity toward (S)-6-chloro-5-hydroxy-3-oxohexanoate ((S)-CHOH), respectively. In the asymmetric reduction, mut-Phe145Tyr/Thr152Ser catalyzed 500 g L−1 of (S)-CHOH to produce (3R,5S)-CDHH with >99% yield and >99% e.e., and the highest space-time yield achieved at 752.76 mmol L−1 h−1 g−1 wet cell weight within 8 h bioconversion. This study provides a foundation for the preparation of (3R,5S)-CDHH by carbonyl reductase.
中文翻译:

羰基还原酶活性的改进的生物生产叔丁基(3 - [R,5小号)- 6-氯-3,5-二羟基
叔丁基(3 - [R,5小号)- 6-氯-3,5-二羟基- ((3 - [R,5小号)-CDHH)是罗苏伐他汀的侧链合成的关键手性中间体。在这项研究中,随机诱变,位点饱和诱变和组合诱变方法被用于提高合成的立体选择性短链羰基还原酶(SCR)的活性以制备(3 R,5 S)-CDHH。经高通量筛选和高效液相色谱法筛选后,获得了mut-Phe145Met / Thr152Ser和mut-Phe145Tyr / Thr152Ser,突变体的酶活性比亲本酶提高了1.60倍和1.91倍,分别。催化效率(ķ猫/ ķ MUT-Phe145Met / Thr152Ser和MUT-Phe145Tyr / Thr152Ser的米)显示出在朝向(初始活性5.11- 8.07和倍改进小号)- 6-氯-5-羟基-3-氧代己酸乙酯( (S)-CHOH)。在不对称还原中,mut-Phe145Tyr / Thr152Ser催化500 g L -1的(S)-CHOH产生(3 R,5 S)-CDHH具有> 99%的产率和> 99%ee值,并在752.76毫摩尔大号实现的最高的时空产率-1 ħ -1 克-1 8小时生物转化中细胞湿重。该研究为羰基还原酶制备(3 R,5 S)-CDHH提供了基础。












































 京公网安备 11010802027423号
京公网安备 11010802027423号