当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Potential Synergy of Chlorine and Potassium and Sodium Elements in Carbonation Enhancement of CaO-Based Sorbents
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2018-07-23 00:00:00 , DOI: 10.1021/acssuschemeng.8b01941 Yongqing Xu , Haoran Ding , Cong Luo , Ying Zheng , Qi Zhang , Xiaoshan Li , Jian Sun 1 , Liqi Zhang
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2018-07-23 00:00:00 , DOI: 10.1021/acssuschemeng.8b01941 Yongqing Xu , Haoran Ding , Cong Luo , Ying Zheng , Qi Zhang , Xiaoshan Li , Jian Sun 1 , Liqi Zhang
Affiliation
|
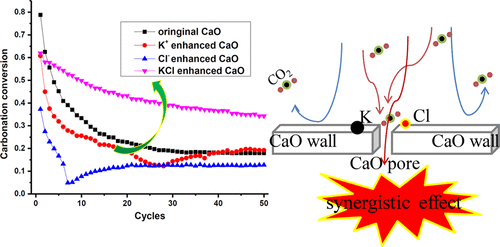
The calcium looping process is well regarded as one of the most prospective technologies for trapping CO2 from flue gas. However, the carbonation conversion of CaO derived from natural Ca precursors decayed drastically during the long-term cycles. Doping has been considered as a promising method to improve the cyclic carbonation performance. However, alkali salt, which derives from abundant natural sustainable resources such as seawater, saline, bittern deposit, and so on, is regarded as a low melting material that can hardly enhance the cyclic carbonation performance of the CaO-based sorbents. In this study, two common alkali salts, sodium and potassium, were doped to investigate the potential enhancing effect of alkali salt on CaO-based sorbents. The cyclic carbonation performance of those sorbents was tested in a simultaneous thermal analyzer (STA), and the enhancing mechanisms were illustrated by scanning electron microscope (SEM), X-ray powder diffraction (XRD), and N2 physical absorption methods. The results illustrated that KCl, NaCl, and K2CO3 boosted the carbonation property of CaO markedly, while KOH, NaOH, and Na2CO3 were detrimental to the sorbents. The synergistic effect of K+, Na+, and Cl– can improve the cyclic carbonation performance of CaO. After 50 cycles, the carbonation conversions of CaO modified by KCl or NaCl were about twice that of unmodified CaO. In general, the KCl and NaCl are promising dopants that can markedly enhance the carbonation property of CaO-based sorbents.
中文翻译:

氯,钾和钠元素在碳酸钙基吸附剂碳酸化增强中的潜在协同作用
钙环化工艺是公认的捕集CO 2的最有前景的技术之一来自烟气。然而,在长期循环中,源自天然Ca前体的CaO的碳酸化转化急剧下降。掺杂已被认为是改善环状碳酸化性能的有前途的方法。然而,碱金属盐被认为是一种低熔点材料,它几乎不能增强CaO基吸附剂的环状碳酸化性能,而碱金属盐是从丰富的自然可持续资源(例如海水,盐水,卤水等)中获得的。在这项研究中,掺杂了两种常见的碱金属盐,钠和钾,以研究碱金属盐对基于CaO的吸附剂的潜在增强作用。在同时热分析仪(STA)中测试了这些吸附剂的环状碳酸化性能,并通过扫描电子显微镜(SEM)阐明了其增强机理,2种物理吸收方法。结果表明,KCl,NaCl和K 2 CO 3显着提高了CaO的碳酸化性能,而KOH,NaOH和Na 2 CO 3则对吸附剂有害。K的协同效应+,钠+和Cl -可以提高CaO的环状碳酸化性能。在50个循环之后,用KCl或NaCl改性的CaO的碳酸化转化率大约是未改性的CaO的碳酸化转化率的两倍。通常,KCl和NaCl是有前途的掺杂剂,可以显着增强CaO基吸附剂的碳酸化性能。
更新日期:2018-07-23
中文翻译:

氯,钾和钠元素在碳酸钙基吸附剂碳酸化增强中的潜在协同作用
钙环化工艺是公认的捕集CO 2的最有前景的技术之一来自烟气。然而,在长期循环中,源自天然Ca前体的CaO的碳酸化转化急剧下降。掺杂已被认为是改善环状碳酸化性能的有前途的方法。然而,碱金属盐被认为是一种低熔点材料,它几乎不能增强CaO基吸附剂的环状碳酸化性能,而碱金属盐是从丰富的自然可持续资源(例如海水,盐水,卤水等)中获得的。在这项研究中,掺杂了两种常见的碱金属盐,钠和钾,以研究碱金属盐对基于CaO的吸附剂的潜在增强作用。在同时热分析仪(STA)中测试了这些吸附剂的环状碳酸化性能,并通过扫描电子显微镜(SEM)阐明了其增强机理,2种物理吸收方法。结果表明,KCl,NaCl和K 2 CO 3显着提高了CaO的碳酸化性能,而KOH,NaOH和Na 2 CO 3则对吸附剂有害。K的协同效应+,钠+和Cl -可以提高CaO的环状碳酸化性能。在50个循环之后,用KCl或NaCl改性的CaO的碳酸化转化率大约是未改性的CaO的碳酸化转化率的两倍。通常,KCl和NaCl是有前途的掺杂剂,可以显着增强CaO基吸附剂的碳酸化性能。






























 京公网安备 11010802027423号
京公网安备 11010802027423号