Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereodynamic Analysis of New Atropisomeric 4,7-Di(naphthalen-1-yl)-5,6-dinitro-1H-indoles
Synlett ( IF 1.7 ) Pub Date : 2018-07-20 , DOI: 10.1055/s-0037-1609908 Giovanni Petrillo 1 , Michele Mancinelli 2 , Angela Pagano 1 , Emanuela Marotta 2 , Andrea Mazzanti 2 , Cinzia Tavani 1
Synlett ( IF 1.7 ) Pub Date : 2018-07-20 , DOI: 10.1055/s-0037-1609908 Giovanni Petrillo 1 , Michele Mancinelli 2 , Angela Pagano 1 , Emanuela Marotta 2 , Andrea Mazzanti 2 , Cinzia Tavani 1
Affiliation
|
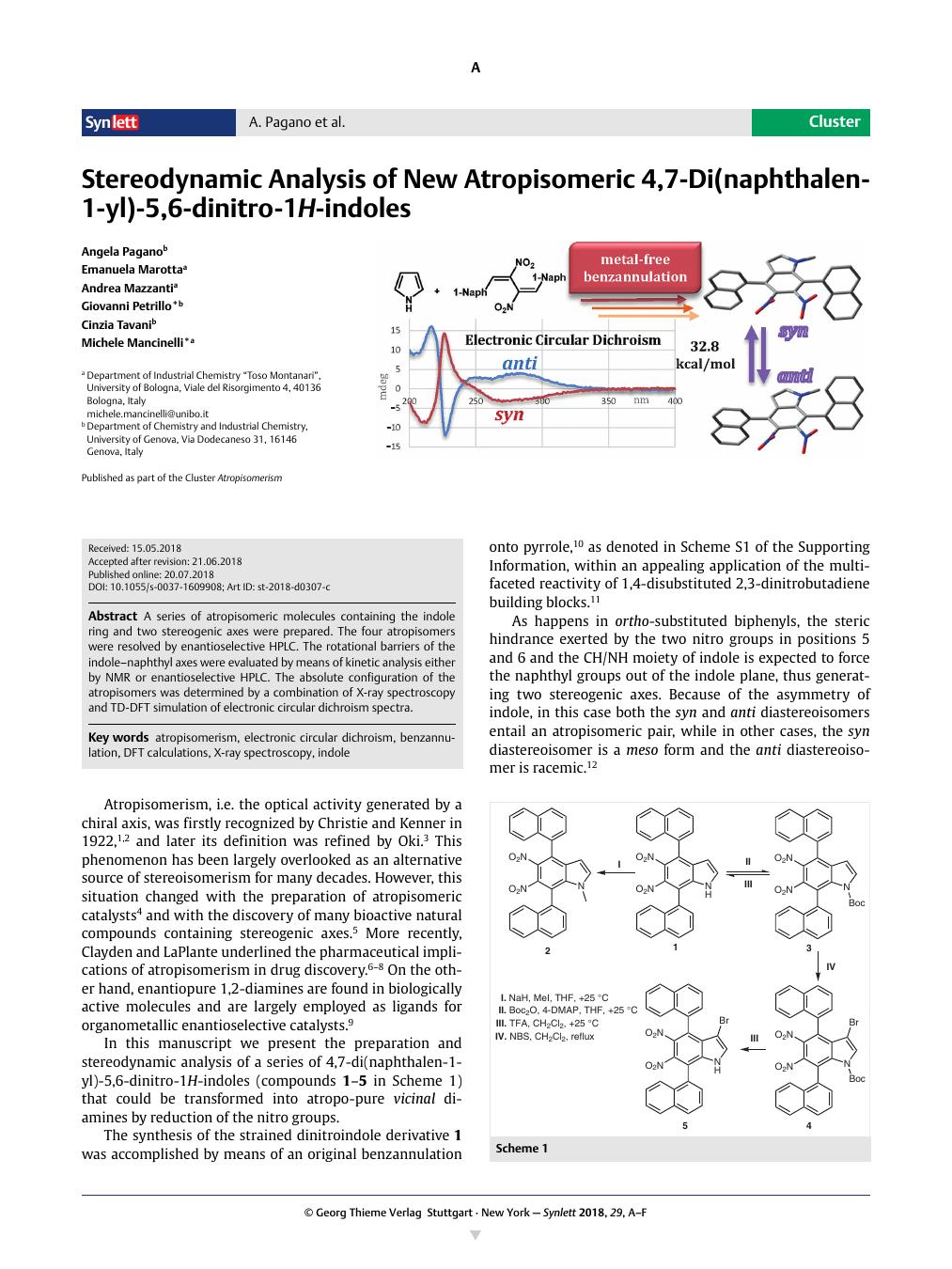
A series of atropisomeric molecules containing the indole ring and two stereogenic axes were prepared. The four atropisomers were resolved by enantioselective HPLC. The rotational barriers of the indole–naphthyl axes were evaluated by means of kinetic analysis either by NMR or enantioselective HPLC. The absolute configuration of the atropisomers was determined by a combination of X-ray spectroscopy and TD-DFT simulation of electronic circular dichroism spectra.
中文翻译:

新型阻转异构体 4,7-Di(naphthalen-1-yl)-5,6-dinitro-1H-indoles 的立体动力学分析
制备了一系列含有吲哚环和两个立体轴的阻转异构分子。四种阻转异构体通过对映选择性 HPLC 进行拆分。吲哚-萘基轴的旋转障碍通过核磁共振或对映选择性 HPLC 的动力学分析进行评估。阻转异构体的绝对构型通过 X 射线光谱和电子圆二色光谱的 TD-DFT 模拟的组合确定。
更新日期:2018-07-20
中文翻译:

新型阻转异构体 4,7-Di(naphthalen-1-yl)-5,6-dinitro-1H-indoles 的立体动力学分析
制备了一系列含有吲哚环和两个立体轴的阻转异构分子。四种阻转异构体通过对映选择性 HPLC 进行拆分。吲哚-萘基轴的旋转障碍通过核磁共振或对映选择性 HPLC 的动力学分析进行评估。阻转异构体的绝对构型通过 X 射线光谱和电子圆二色光谱的 TD-DFT 模拟的组合确定。

































 京公网安备 11010802027423号
京公网安备 11010802027423号