Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of Catalyst Processing on the Activity and Stability of Pt–Ni Nanoframe Electrocatalysts
ACS Nano ( IF 15.8 ) Pub Date : 2018-07-20 00:00:00 , DOI: 10.1021/acsnano.8b04674 Shouping Chen 1 , Zhiqiang Niu , Chenlu Xie , Mengyu Gao 1 , Minliang Lai , Mufan Li 1 , Peidong Yang 1, 2
ACS Nano ( IF 15.8 ) Pub Date : 2018-07-20 00:00:00 , DOI: 10.1021/acsnano.8b04674 Shouping Chen 1 , Zhiqiang Niu , Chenlu Xie , Mengyu Gao 1 , Minliang Lai , Mufan Li 1 , Peidong Yang 1, 2
Affiliation
|
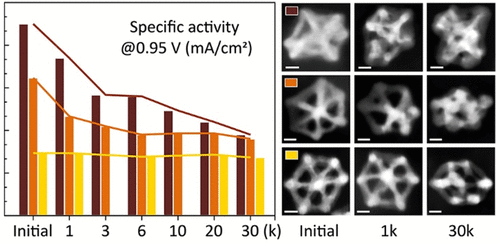
Pt-based alloys have shown great promise as cathodic catalysts for cost-effective proton-exchange membrane fuel cells. Post-synthesis treatment has been recognized as a critical step to improve the catalytic performance of Pt-based alloys. Here, we present the effects of catalyst processing on the catalytic behavior of Pt–Ni nanoframe electrocatalysts in oxygen reduction reaction. The Pt–Ni nanoframes were made by corroding the Ni-rich phase from solid rhombic dodecahedral particles. A total of three different corrosion procedures were compared. Among them, electrochemical corrosion led to the highest initial specific activity (1.35 mA cm–2 at 0.95 V versus reversible hydrogen electrode) by retaining more Ni in the nanoframes. However, the high activity gradually went down in a subsequent stability test due to continuous Ni loss and concomitant surface reconstruction. On the other hand, the best stability was achieved by a more-aggressive corrosion using oxidative nitric acid. Although the initial activity was compromised, this procedure imparted a less-defective surface, and thus, the specific activity dropped by only 7% over 30 000 potential cycles. These results indicate a delicate trade-off between the activity and stability of Pt–Ni nanoframe electrocatalysts. The obtained understanding of how to balance the activity–stability trade-off via catalyst processing can be generalized to other Pt-based alloys.
中文翻译:

催化剂工艺对Pt-Ni纳米框架电催化剂活性和稳定性的影响
铂基合金作为具有成本效益的质子交换膜燃料电池的阴极催化剂已显示出广阔的前景。合成后处理已被认为是改善Pt基合金催化性能的关键步骤。在这里,我们介绍了催化剂处理对Pt-Ni纳米框架电催化剂在氧还原反应中的催化行为的影响。Pt-Ni纳米框架是通过腐蚀固态菱形十二面体颗粒中的富镍相而制成的。总共比较了三种不同的腐蚀程序。其中,电化学腐蚀导致最高的初始比活度(相对于0.95 V,1.35 mA cm –2可逆氢电极)通过在纳米框架中保留更多的镍。然而,由于连续的镍损失和伴随的表面重建,高活性在随后的稳定性测试中逐渐下降。另一方面,最好的稳定性是通过使用氧化性硝酸进行的更剧烈的腐蚀而获得的。尽管初始活性受到损害,但该程序的表面缺陷较少,因此,在3万个潜在循环中,比活性仅下降了7%。这些结果表明,Pt-Ni纳米框架电催化剂的活性和稳定性之间存在微妙的折衷。对如何通过催化剂处理平衡活性与稳定性之间的权衡取舍的理解可以推广到其他基于Pt的合金中。
更新日期:2018-07-20
中文翻译:

催化剂工艺对Pt-Ni纳米框架电催化剂活性和稳定性的影响
铂基合金作为具有成本效益的质子交换膜燃料电池的阴极催化剂已显示出广阔的前景。合成后处理已被认为是改善Pt基合金催化性能的关键步骤。在这里,我们介绍了催化剂处理对Pt-Ni纳米框架电催化剂在氧还原反应中的催化行为的影响。Pt-Ni纳米框架是通过腐蚀固态菱形十二面体颗粒中的富镍相而制成的。总共比较了三种不同的腐蚀程序。其中,电化学腐蚀导致最高的初始比活度(相对于0.95 V,1.35 mA cm –2可逆氢电极)通过在纳米框架中保留更多的镍。然而,由于连续的镍损失和伴随的表面重建,高活性在随后的稳定性测试中逐渐下降。另一方面,最好的稳定性是通过使用氧化性硝酸进行的更剧烈的腐蚀而获得的。尽管初始活性受到损害,但该程序的表面缺陷较少,因此,在3万个潜在循环中,比活性仅下降了7%。这些结果表明,Pt-Ni纳米框架电催化剂的活性和稳定性之间存在微妙的折衷。对如何通过催化剂处理平衡活性与稳定性之间的权衡取舍的理解可以推广到其他基于Pt的合金中。






























 京公网安备 11010802027423号
京公网安备 11010802027423号