当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CO2 Electroreduction in Ionic Liquids: A Review
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2018-08-28 , DOI: 10.1002/cjoc.201800252
Jianpeng Feng 1, 2 , Shaojuan Zeng 1 , Jiaqi Feng 1, 2 , Haifeng Dong 1 , Xiangping Zhang 1, 2
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2018-08-28 , DOI: 10.1002/cjoc.201800252
Jianpeng Feng 1, 2 , Shaojuan Zeng 1 , Jiaqi Feng 1, 2 , Haifeng Dong 1 , Xiangping Zhang 1, 2
Affiliation
|
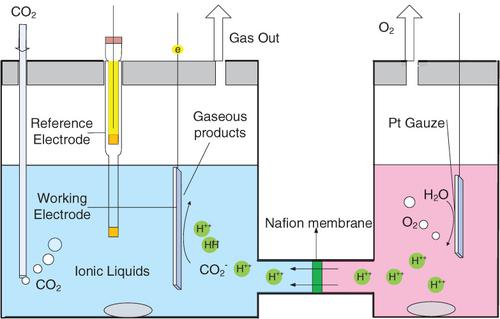
The increasing emission of carbon dioxide (CO2) caused by the unrestrained consumption of fossil fuels in recent hundreds of years, has caused global environmental and social problems. Meanwhile, CO2 is a cheap, abundant and renewable C1‐feedstock, which can be converted into alcohols, ethers, acids and other value‐added chemicals. Compared with the thermal reactions, electrochemical reduction of CO2 is more attractive because of its advantages by using the seasonal, geographical and intermittent energy (tide, wind and solar) under mild conditions. In recent years, taking ionic liquids (ILs) as electrolytes in the CO2 electrochemical reduction reaction has been paid much more attention due to the advantages of lowering the overpotential of CO2 electroreduction and improving the Faradaic efficiency. In this paper, we summarized the recent progresses of electrochemical reduction of CO2 in ILs electrolytes, and analyzed the reaction mechanism of CO2 reaction in the electrode‐electrolyte interface region by experimental and simulation methods. Finally, the research which needs to be highlighted in this area was proposed.
中文翻译:

离子液体中的二氧化碳电还原:综述
近百年来,由于不受限制地消耗化石燃料而导致的二氧化碳(CO 2)排放量增加,已经引起了全球环境和社会问题。同时,CO 2是一种廉价,丰富且可再生的C1原料,可以转化为醇,醚,酸和其他增值化学品。与热反应相比,由于在温和条件下使用季节性,地理和间歇性能量(潮汐,风能和太阳能),CO 2的电化学还原更具吸引力,因为它具有优势。近年来,以离子液体(ILs)为电解质在CO 2中电化学还原反应由于降低了CO 2电还原的过电位和提高了法拉第效率的优点而受到了更多的关注。在本文中,我们总结了ILs电解质中电化学还原CO 2的最新进展,并通过实验和模拟方法分析了CO 2反应在电极-电解质界面区域的反应机理。最后,提出了该领域需要重点研究的问题。
更新日期:2018-08-28
中文翻译:

离子液体中的二氧化碳电还原:综述
近百年来,由于不受限制地消耗化石燃料而导致的二氧化碳(CO 2)排放量增加,已经引起了全球环境和社会问题。同时,CO 2是一种廉价,丰富且可再生的C1原料,可以转化为醇,醚,酸和其他增值化学品。与热反应相比,由于在温和条件下使用季节性,地理和间歇性能量(潮汐,风能和太阳能),CO 2的电化学还原更具吸引力,因为它具有优势。近年来,以离子液体(ILs)为电解质在CO 2中电化学还原反应由于降低了CO 2电还原的过电位和提高了法拉第效率的优点而受到了更多的关注。在本文中,我们总结了ILs电解质中电化学还原CO 2的最新进展,并通过实验和模拟方法分析了CO 2反应在电极-电解质界面区域的反应机理。最后,提出了该领域需要重点研究的问题。

































 京公网安备 11010802027423号
京公网安备 11010802027423号