当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric synthesis of homoallylic amines via 1,2-addition of Grignard reagent to aliphatic N-phosphonyl hemiaminal
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2016-01-11 12:43:36 Shuo Qiao, Suresh Pindi, Preston T. Spigener, Bo Jiang, Guigen Li
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2016-01-11 12:43:36 Shuo Qiao, Suresh Pindi, Preston T. Spigener, Bo Jiang, Guigen Li
|
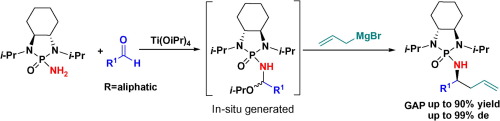
A general method for asymmetric synthesis of alkylated homoallylic amines was developed via a one-pot three-component reaction of easily available N-phosphonyl amides, aliphatic aldehydes, and allylic Grignard reagents. As anticipated the reaction proceeds through six-membered chelation controlled mechanism, allowing 1,2-nucleophilic addition to directly give chiral homoallylic amines with high yields and excellent diastereoselectivity.
中文翻译:

通过格氏试剂与脂肪族N-膦酰基半缩醛的1,2-加成反应合成不对称胺
通过容易获得的N-膦酰基酰胺,脂肪族醛和烯丙基格利雅试剂的一锅三组分反应,开发了一种不对称合成烷基化均烯丙基胺的通用方法。如所预期的,反应通过六元螯合控制机制进行,允许1,2-亲核加成以高收率和优异的非对映选择性直接得到手性均烯丙基胺。
更新日期:2016-01-12
中文翻译:

通过格氏试剂与脂肪族N-膦酰基半缩醛的1,2-加成反应合成不对称胺
通过容易获得的N-膦酰基酰胺,脂肪族醛和烯丙基格利雅试剂的一锅三组分反应,开发了一种不对称合成烷基化均烯丙基胺的通用方法。如所预期的,反应通过六元螯合控制机制进行,允许1,2-亲核加成以高收率和优异的非对映选择性直接得到手性均烯丙基胺。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号