当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Antibody Fucosylation Lowers the FcγRIIIa/CD16a Affinity by Limiting the Conformations Sampled by the N162-Glycan
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-07-17 00:00:00 , DOI: 10.1021/acschembio.8b00342 Daniel J. Falconer 1 , Ganesh P. Subedi 1 , Aaron M. Marcella 1 , Adam W. Barb 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-07-17 00:00:00 , DOI: 10.1021/acschembio.8b00342 Daniel J. Falconer 1 , Ganesh P. Subedi 1 , Aaron M. Marcella 1 , Adam W. Barb 1
Affiliation
|
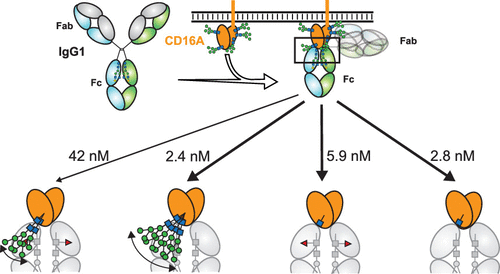
Therapeutic monoclonal antibodies (mAbs) are largely based on the immunoglobulin G1 (IgG1) scaffold, and many elicit a cytotoxic cell-mediated response by binding Fc γ receptors. Core fucosylation, a prevalent modification to the asparagine (N)-linked carbohydrate on the IgG1 crystallizable fragment (Fc), decreases the Fc γ receptor IIIa (CD16a) binding affinity and mAb efficacy. We determined IgG1 Fc fucosylation reduced the CD16a affinity by 1.7 ± 0.1 kcal/mol when compared to that of afucosylated IgG1 Fc; however, CD16a N-glycan truncation decreased this penalty by 1.2 ± 0.1 kcal/mol or 70%. Fc fucosylation restricted the manifold of conformations sampled by displacing the CD16a Asn162-glycan that impinges upon the linkage between the α-mannose(1–6)β-mannose residues and promoted contacts with the IgG Tyr296 residue. Fucosylation also impacted the IgG1 Fc structure as indicated by changes in resonance frequencies and nuclear spin relaxation observed by solution nuclear magnetic resonance spectroscopy. The effects of fucosylation on IgG1 Fc may account for the remaining 0.5 ± 0.1 kcal/mol penalty of fucosylated IgG1 Fc binding CD16a when compared to that of afucosylated IgG1 Fc. Our results indicated the CD16a Asn162-glycan modulates the antibody affinity indirectly by reducing the volume sampled, as opposed to a direct mechanism with intermolecular glycan–glycan contacts previously proposed to stabilize this system. Thus, antibody engineering to enhance intermolecular glycan–glycan contacts will likely provide limited improvement, and future designs should maximize the affinity by maintaining the CD16a Asn162-glycan conformational heterogeneity.
中文翻译:

抗体岩藻糖基化通过限制N162-聚糖取样的构象降低FcγRIIIa/ CD16a的亲和力
治疗性单克隆抗体(mAbs)很大程度上基于免疫球蛋白G1(IgG1)支架,许多单克隆抗体通过结合Fcγ受体引起细胞毒性细胞介导的反应。核心岩藻糖基化是对IgG1可结晶片段(Fc)上天冬酰胺(N)-连接的碳水化合物的普遍修饰,会降低Fcγ受体IIIa(CD16a)的结合亲和力和mAb功效。我们确定,与无岩藻糖基化的IgG1 Fc相比,IgG1 Fc岩藻糖基化使CD16a亲和力降低了1.7±0.1 kcal / mol。然而,CD16a N-糖基团截短可将这种损失降低1.2±0.1 kcal / mol或70%。Fc岩藻糖基化作用限制了通过置换CD16a Asn162-聚糖(影响α-甘露糖(1-6)β-甘露糖残基之间的连接并促进与IgG Tyr296残基的接触)所采样的构象的多样性。岩藻糖基化还影响IgG1 Fc结构,这通过溶液核磁共振波谱观察到的共振频率变化和核自旋弛豫来表明。与岩藻糖基化IgG1 Fc相比,岩藻糖基化对IgG1 Fc的影响可解释岩藻糖基化IgG1 Fc结合CD16a的剩余0.5±0.1 kcal / mol罚分。我们的结果表明,CD16a Asn162-聚糖通过减少采样量间接调节抗体亲和力,这与先前提出的稳定分子间聚糖-聚糖接触的直接机制相反。因此,用于增强分子间聚糖与聚糖接触的抗体工程可能会提供有限的改进,未来的设计应通过保持CD16a Asn162-聚糖构象异质性来最大化亲和力。
更新日期:2018-07-17
中文翻译:

抗体岩藻糖基化通过限制N162-聚糖取样的构象降低FcγRIIIa/ CD16a的亲和力
治疗性单克隆抗体(mAbs)很大程度上基于免疫球蛋白G1(IgG1)支架,许多单克隆抗体通过结合Fcγ受体引起细胞毒性细胞介导的反应。核心岩藻糖基化是对IgG1可结晶片段(Fc)上天冬酰胺(N)-连接的碳水化合物的普遍修饰,会降低Fcγ受体IIIa(CD16a)的结合亲和力和mAb功效。我们确定,与无岩藻糖基化的IgG1 Fc相比,IgG1 Fc岩藻糖基化使CD16a亲和力降低了1.7±0.1 kcal / mol。然而,CD16a N-糖基团截短可将这种损失降低1.2±0.1 kcal / mol或70%。Fc岩藻糖基化作用限制了通过置换CD16a Asn162-聚糖(影响α-甘露糖(1-6)β-甘露糖残基之间的连接并促进与IgG Tyr296残基的接触)所采样的构象的多样性。岩藻糖基化还影响IgG1 Fc结构,这通过溶液核磁共振波谱观察到的共振频率变化和核自旋弛豫来表明。与岩藻糖基化IgG1 Fc相比,岩藻糖基化对IgG1 Fc的影响可解释岩藻糖基化IgG1 Fc结合CD16a的剩余0.5±0.1 kcal / mol罚分。我们的结果表明,CD16a Asn162-聚糖通过减少采样量间接调节抗体亲和力,这与先前提出的稳定分子间聚糖-聚糖接触的直接机制相反。因此,用于增强分子间聚糖与聚糖接触的抗体工程可能会提供有限的改进,未来的设计应通过保持CD16a Asn162-聚糖构象异质性来最大化亲和力。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号