JAMA Neurology ( IF 20.4 ) Pub Date : 2018-10-01 , DOI: 10.1001/jamaneurol.2018.1836 Ming-Kai Chen 1 , Adam P. Mecca 2 , Mika Naganawa 1 , Sjoerd J. Finnema 1 , Takuya Toyonaga 1 , Shu-fei Lin 1 , Soheila Najafzadeh 1 , Jim Ropchan 1 , Yihuan Lu 1 , Julia W. McDonald 2 , Hannah R. Michalak 2 , Nabeel B. Nabulsi 1 , Amy F. T. Arnsten 2 , Yiyun Huang 1 , Richard E. Carson 1 , Christopher H. van Dyck 2
|
Importance Synaptic loss is well established as the major structural correlate of cognitive impairment in Alzheimer disease (AD). The ability to measure synaptic density in vivo could accelerate the development of disease-modifying treatments for AD. Synaptic vesicle glycoprotein 2A is an essential vesicle membrane protein expressed in virtually all synapses and could serve as a suitable target for synaptic density.
Objective To compare hippocampal synaptic vesicle glycoprotein 2A (SV2A) binding in participants with AD and cognitively normal participants using positron emission tomographic (PET) imaging.
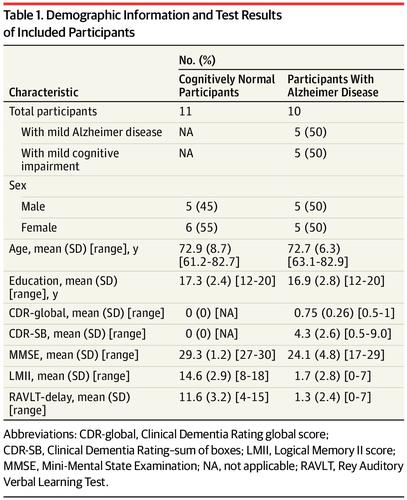
Design, Setting, and Participants This cross-sectional study recruited 10 participants with AD and 11 participants who were cognitively normal between November 2015 and June 2017. We hypothesized a reduction in hippocampal SV2A binding in AD, based on the early degeneration of entorhinal cortical cell projections to the hippocampus (via the perforant path) and hippocampal SV2A reductions that had been observed in postmortem studies. Participants underwent high-resolution PET scanning with ((R)-1-((3-(11C-methyl-11C)pyridin-4-yl)methyl)-4-(3,4,5-trifluorophenyl)pyrrolidin-2-one), a compound more commonly known as 11C-UCB-J, for SV2A. They also underwent high-resolution PET scanning with carbon 11–labeled Pittsburgh Compound B (11C-PiB) for β-amyloid, magnetic resonance imaging, and cognitive and neurologic evaluation.
Main Outcomes and Measures Outcomes were 11C-UCB-J–specific binding (binding potential [BPND]) via PET imaging in brain regions of interest in participants with AD and participants who were cognitively normal.
Results Ten participants with AD (5 male and 5 female; mean [SD] age, 72.7 [6.3] years; 10 [100%] β-amyloid positive) were compared with 11 participants who were cognitively normal (5 male and 6 female; mean [SD] age, 72.9 [8.7] years; 11 [100%] β-amyloid negative). Participants with AD spanned the disease stages from amnestic mild cognitive impairment (n = 5) to mild dementia (n = 5). Participants with AD had significant reduction in hippocampal SV2A specific binding (41%) compared with cognitively normal participants, as assessed by 11C-UCB-J–PET BPND (cognitively normal participants: mean [SD] BPND, 1.47 [0.37]; participants with AD: 0.87 [0.50]; P = .005). These reductions remained significant after correction for atrophy (ie, partial volume correction; participants who were cognitively normal: mean [SD], 2.71 [0.46]; participants with AD: 2.15 [0.55]; P = .02). Hippocampal SV2A-specific binding BPND was correlated with a composite episodic memory score in the overall sample (R = 0.56; P = .01).
Conclusions and Relevance To our knowledge, this is the first study to investigate synaptic density in vivo in AD using 11C-UCB-J–PET imaging. This approach may provide a direct measure of synaptic density, and it therefore holds promise as an in vivo biomarker for AD and as an outcome measure for trials of disease-modifying therapies, particularly those targeted at the preservation and restoration of synapses.
中文翻译:

突触囊泡糖蛋白2A正电子发射断层成像成像评估阿尔茨海默病的突触密度。
重要性 突触丧失已被确立为阿尔茨海默病(AD)认知障碍的主要结构相关因素。在体内测量突触密度的能力可以加速针对AD的疾病缓解疗法的发展。突触囊泡糖蛋白2A是实际上在所有突触中表达的必需囊泡膜蛋白,可作为突触密度的合适靶标。
目的 使用正电子发射断层扫描(PET)成像比较AD参与者和认知正常参与者的海马突触囊泡糖蛋白2A(SV2A)结合。
设计,设置和参加者 该横断面研究招募了10名AD参与者和11名在2015年11月至2017年6月之间认知正常的参与者。我们基于内嗅皮质细胞的早期变性,假设AD的海马SV2A结合减少。在验尸研究中观察到的海马投射(通过穿孔路径)和海马SV2A减少。参加者接受了((R) -1-((3-(11C-甲基-11C)吡啶-4-基)甲基)-4-(3,4,5-三氟苯基)吡咯烷酮-2-的高分辨率PET扫描一种),一种通常被称为11 C-UCB-J的SV2A化合物。他们还用碳11标记的匹兹堡化合物B进行了高分辨率PET扫描(11C-PiB)用于β淀粉样蛋白,磁共振成像以及认知和神经系统评估。
主要结果和措施 结果是通过PET成像在AD参与者和认知正常的参与者感兴趣的大脑区域中通过PET成像产生11种C-UCB-J特异性结合(结合潜力[ BP ND ])。
结果将 10名患有AD的参与者(5名男性和5名女性;平均[SD]年龄为72.7 [6.3]岁; 10 [100%]β-淀粉样蛋白阳性)与11名认知正常的参与者(5名男性和6名女性; 5名女性)进行了比较。平均[SD]年龄为72.9 [8.7]岁; 11 [100%]β-淀粉样蛋白阴性)。患有AD的参与者跨越了从轻度轻度认知障碍(n = 5)到轻度痴呆(n = 5)的疾病阶段。由11 C-UCB-J-PET BP ND评估(认知正常参与者:平均[SD] BP ND,1.47 [0.37] ),与认知正常参与者相比,AD参与者的海马SV2A特异性结合显着降低(41%)。; AD参与者:0.87 [0.50];P = .005)。萎缩校正后(即部分容积校正;认知正常的参与者:平均值[SD],2.71 [0.46]; AD参与者:2.15 [0.55];P = .02),这些减少仍然很明显。海马SV2A特异性结合BP ND与总体样本中的复合情节记忆评分相关(R = 0.56;P = 0.01)。
结论和相关性 据我们所知,这是第一项使用11 C-UCB-J-PET成像研究AD体内突触密度的研究。这种方法可以提供突触密度的直接量度,因此有望作为AD的体内生物标记物,并作为疾病修饰疗法(尤其是针对突触的保存和恢复的疗法)试验的结果指标。































 京公网安备 11010802027423号
京公网安备 11010802027423号