Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical investigation of the decarboxylation and decarbonylation mechanism of propanoic acid over a Ru(0001) model surface
Journal of Catalysis ( IF 6.5 ) Pub Date : 2015-04-24 05:55:09 Jianmin Lu , Muhammad Faheem , Sina Behtash , Andreas Heyden
Journal of Catalysis ( IF 6.5 ) Pub Date : 2015-04-24 05:55:09 Jianmin Lu , Muhammad Faheem , Sina Behtash , Andreas Heyden
|
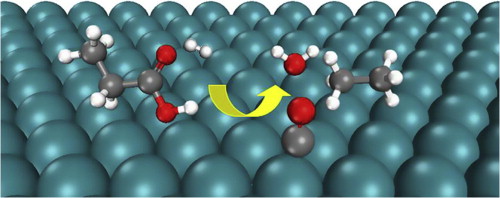
The hydrodeoxygenation of organic acids is often found to be a rate-controlling process during upgrading of biomass feedstocks into fuels. We developed a microkinetic model based on data obtained from density functional theory calculations for the decarboxylation and decarbonylation mechanisms of propanoic acid (CH3CH2COOH) over a Ru(0001) model surface. The model predicts that the decarbonylation mechanism is two orders of magnitude faster than the decarboxylation mechanism. The most favorable decarbonylation pathway proceeds via removal of the acid –OH group to produce propanoyl (CH3CH2CO) followed by C–CO bond scission of propanoyl to produce CH3CH2 and CO. Finally, CH3CH2 is hydrogenated to CH3CH3. Dehydrogenation reactions that have been observed to be important over Pd catalysts play no role over Ru(0001), and a sensitivity analysis indicates that removal of the acid –OH group is the rate-controlling step in the deoxygenation. Overall, our results suggest that to improve the Ru catalyst performance for the decarbonylation of organic acids, the free site coverage needs to be increased by, for example, adding a catalyst promoter that decreases the hydrogen and CO adsorption strength (without significantly affecting the C–OH bond scission rate), or by raising the reaction temperature and operating at relatively low CO and H2 partial pressures.
中文翻译:

Ru(0001)模型表面丙酸脱羧脱羰机理的理论研究
在将生物质原料转化为燃料的过程中,经常发现有机酸的加氢脱氧是一个速率控制过程。我们基于从密度泛函理论计算获得的数据开发了一个微动力学模型,用于在Ru(0001)模型表面上丙酸(CH 3 CH 2 COOH)的脱羧和脱羰机理。该模型预测脱羰机理比脱羧机理快两个数量级。最有利的脱羰途径是通过除去酸-OH基团生成丙酰基(CH 3 CH 2 CO),然后进行丙酰基的C-CO键断裂,生成CH 3 CH 2和CO。3 CH 2氢化成CH 3 CH 3。观察到对Pd催化剂而言重要的脱氢反应对Ru(0001)不起任何作用,敏感性分析表明,酸-OH基的去除是脱氧过程中的速率控制步骤。总体而言,我们的结果表明,要提高Ru催化剂对有机酸脱羰的性能,需要增加自由位点的覆盖度,例如,通过添加降低氢和CO吸附强度(而不会显着影响C -OH键断裂速率),或通过提高反应温度并在相对较低的CO和H 2分压下运行。
更新日期:2015-04-24
中文翻译:

Ru(0001)模型表面丙酸脱羧脱羰机理的理论研究
在将生物质原料转化为燃料的过程中,经常发现有机酸的加氢脱氧是一个速率控制过程。我们基于从密度泛函理论计算获得的数据开发了一个微动力学模型,用于在Ru(0001)模型表面上丙酸(CH 3 CH 2 COOH)的脱羧和脱羰机理。该模型预测脱羰机理比脱羧机理快两个数量级。最有利的脱羰途径是通过除去酸-OH基团生成丙酰基(CH 3 CH 2 CO),然后进行丙酰基的C-CO键断裂,生成CH 3 CH 2和CO。3 CH 2氢化成CH 3 CH 3。观察到对Pd催化剂而言重要的脱氢反应对Ru(0001)不起任何作用,敏感性分析表明,酸-OH基的去除是脱氧过程中的速率控制步骤。总体而言,我们的结果表明,要提高Ru催化剂对有机酸脱羰的性能,需要增加自由位点的覆盖度,例如,通过添加降低氢和CO吸附强度(而不会显着影响C -OH键断裂速率),或通过提高反应温度并在相对较低的CO和H 2分压下运行。































 京公网安备 11010802027423号
京公网安备 11010802027423号