当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isofunctional Enzymes PAD1 and UbiX Catalyze Formation of a Novel Cofactor Required by Ferulic Acid Decarboxylase and 4-Hydroxy-3-polyprenylbenzoic Acid Decarboxylase
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2015-02-03 00:00:00 , DOI: 10.1021/cb5008103 Fengming Lin 1 , Kyle L. Ferguson 1 , David R. Boyer 1 , Xiaoxia Nina Lin 1 , E. Neil G. Marsh 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2015-02-03 00:00:00 , DOI: 10.1021/cb5008103 Fengming Lin 1 , Kyle L. Ferguson 1 , David R. Boyer 1 , Xiaoxia Nina Lin 1 , E. Neil G. Marsh 1
Affiliation
|
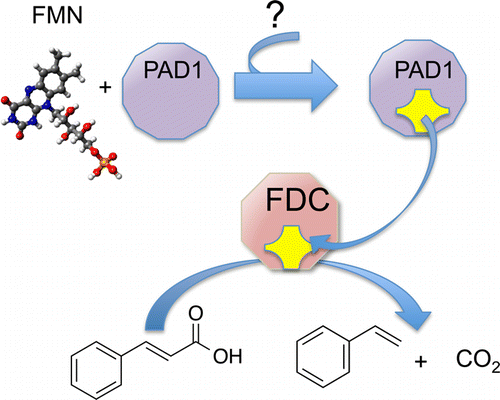
The decarboxylation of antimicrobial aromatic acids such as phenylacrylic acid (cinnamic acid) and ferulic acid by yeast requires two enzymes described as phenylacrylic acid decarboxylase (PAD1) and ferulic acid decarboxylase (FDC). These enzymes are of interest for various biotechnological applications, such as the production of chemical feedstocks from lignin under mild conditions. However, the specific role of each protein in catalyzing the decarboxylation reaction remains unknown. To examine this, we have overexpressed and purified both PAD1 and FDC from E. coli. We demonstrate that PAD1 is a flavin mononucleotide (FMN)-containing protein. However, it does not function as a decarboxylase. Rather, PAD1 catalyzes the formation of a novel, diffusible cofactor required by FDC for decarboxylase activity. Coexpression of FDC and PAD1 results in the production of FDC with high levels cofactor bound. Holo-FDC catalyzes the decarboxylation of phenylacrylic acid, coumaric acid and ferulic acid with apparent kcat ranging from 1.4–4.6 s–1. The UV-visible and mass spectra of the cofactor indicate that it appears to be a novel, modified form of reduced FMN; however, its instability precluded determination of its structure. The E. coli enzymes UbiX and UbiD are related by sequence to PAD1 and FDC respectively and are involved in the decarboxylation of 4-hydroxy-3-octaprenylbenzoic acid, an intermediate in ubiquinone biosynthesis. We found that endogenous UbiX can also activate FDC. This implies that the same cofactor is required for decarboxylation of 4-hydroxy-3-polyprenylbenzoic acid by UbiD and suggests a wider role for this cofactor in metabolism.
中文翻译:

同工酶PAD1和UbiX催化阿魏酸脱羧酶和4-羟基-3-聚异戊烯基苯甲酸脱羧酶所需的新型辅因子的形成
酵母使抗菌性芳族酸(如苯基丙烯酸(肉桂酸)和阿魏酸)脱羧需要两种酶,分别称为苯基丙烯酸脱羧酶(PAD1)和阿魏酸脱羧酶(FDC)。这些酶对于各种生物技术应用都是有意义的,例如在温和条件下从木质素生产化学原料。但是,每种蛋白质在催化脱羧反应中的特定作用仍是未知的。为了检查这一点,我们从大肠杆菌中过表达并纯化了PAD1和FDC 。我们证明,PAD1是一种含有黄素单核苷酸(FMN)的蛋白质。但是,它不起脱羧酶的作用。相反,PAD1催化FDC进行脱羧酶活性所需的新型可扩散辅因子的形成。FDC和PAD1的共表达导致FDC产生高水平辅因子结合。Holo-FDC催化苯丙烯酸,香豆酸和阿魏酸的脱羧反应,表观k cat范围为1.4–4.6 s –1。辅因子的紫外-可见光谱和质谱表明,它似乎是新颖的还原FMN的修饰形式。但是,它的不稳定性使得无法确定其结构。该大肠杆菌UbiX和UbiD酶在序列上分别与PAD1和FDC相关,并参与4-羟基-3-辛烯基苯甲酸(泛醌生物合成的中间体)的脱羧作用。我们发现内源性UbiX也可以激活FDC。这意味着通过UbiD对4-羟基-3-聚异戊二烯基苯甲酸进行脱羧需要相同的辅助因子,并暗示该辅助因子在代谢中的作用更大。
更新日期:2015-02-03
中文翻译:

同工酶PAD1和UbiX催化阿魏酸脱羧酶和4-羟基-3-聚异戊烯基苯甲酸脱羧酶所需的新型辅因子的形成
酵母使抗菌性芳族酸(如苯基丙烯酸(肉桂酸)和阿魏酸)脱羧需要两种酶,分别称为苯基丙烯酸脱羧酶(PAD1)和阿魏酸脱羧酶(FDC)。这些酶对于各种生物技术应用都是有意义的,例如在温和条件下从木质素生产化学原料。但是,每种蛋白质在催化脱羧反应中的特定作用仍是未知的。为了检查这一点,我们从大肠杆菌中过表达并纯化了PAD1和FDC 。我们证明,PAD1是一种含有黄素单核苷酸(FMN)的蛋白质。但是,它不起脱羧酶的作用。相反,PAD1催化FDC进行脱羧酶活性所需的新型可扩散辅因子的形成。FDC和PAD1的共表达导致FDC产生高水平辅因子结合。Holo-FDC催化苯丙烯酸,香豆酸和阿魏酸的脱羧反应,表观k cat范围为1.4–4.6 s –1。辅因子的紫外-可见光谱和质谱表明,它似乎是新颖的还原FMN的修饰形式。但是,它的不稳定性使得无法确定其结构。该大肠杆菌UbiX和UbiD酶在序列上分别与PAD1和FDC相关,并参与4-羟基-3-辛烯基苯甲酸(泛醌生物合成的中间体)的脱羧作用。我们发现内源性UbiX也可以激活FDC。这意味着通过UbiD对4-羟基-3-聚异戊二烯基苯甲酸进行脱羧需要相同的辅助因子,并暗示该辅助因子在代谢中的作用更大。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号