当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intrinsic, Cancer Cell-Selective Toxicity of Organic Photothermal Nanoagent: A Simple Formulation for Combined Photothermal Chemotherapy of Cancer
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2018-07-13 00:00:00 , DOI: 10.1021/acsami.8b07801 Zhuoxuan Lu 1 , Feng-Ying Huang 1 , Rong Cao 1 , Guang-Hong Tan 1 , Guohui Yi 1 , Nongyue He 2, 3 , Lingfeng Xu 1 , Liming Zhang 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2018-07-13 00:00:00 , DOI: 10.1021/acsami.8b07801 Zhuoxuan Lu 1 , Feng-Ying Huang 1 , Rong Cao 1 , Guang-Hong Tan 1 , Guohui Yi 1 , Nongyue He 2, 3 , Lingfeng Xu 1 , Liming Zhang 1
Affiliation
|
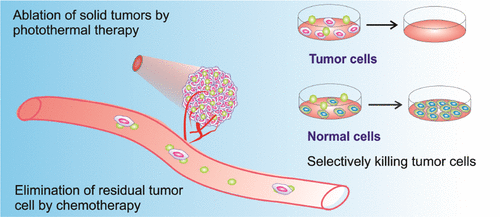
Nano-agent-mediated photothermal therapy (PTT) combined with chemotherapy has been proposed as an effective strategy against cancer. However, chemotherapeutic agents often cause serious side effects. Herein, a novel PTT nanoagent (Cy5.5–MSA–G250) with unanticipated intrinsic tumor-selective cytotoxicity is developed. The Cy5.5–MSA–G250 nanoparticles (NPs) are created by mixing mouse serum albumin (MSA) and coomassie brilliant blue (G250) and then conjugated with cyanine 5.5 (Cy5.5). As expected, Cy5.5–MSA–G250 NPs can efficiently kill cancer cells in vitro and in vivo by PTT. Meanwhile, we accidentally discover that Cy5.5–MSA–G250 have intrinsic specific cytotoxicity against tumor cells but not against normal cells. Moreover, the tumor-specific cytotoxicity of Cy5.5–MSA–G250 is much stronger than that of cytarabine, an FDA-approved anticancer drug. In vivo experiments also prove that Cy5.5–MSA–G250 NPs can effectively eliminate residual tumor cells and prevent metastasis. Further study indicates that selective induction of G1 cell cycle arrest and inhibition of DNA duplication in tumor cells may be the possible mechanism of the tumor cell-selective cytotoxicity of Cy5.5–MSA–G250 NPs. In addition, direct visualization, low systematic toxicity, good biodegradation, and efficient body excretion further make Cy5.5–MSA–G250 NPs attractive for in vivo applications. Taken together, Cy5.5–MSA–G250 NPs are proven to be a promising platform for combined photothermal chemotherapy.
中文翻译:

有机光热纳米剂的内在癌细胞选择性毒性:癌症联合光热化学疗法的简单配方
纳米药物介导的光热疗法(PTT)与化学疗法相结合已被提出作为一种有效的抗癌策略。但是,化学治疗剂通常会引起严重的副作用。在本文中,开发了一种新型PTT纳米剂(Cy5.5–MSA–G250),具有预料不到的内在肿瘤选择性细胞毒性。Cy5.5–MSA–G250纳米颗粒(NPs)是通过将小鼠血清白蛋白(MSA)和考马斯亮蓝(G250)混合,然后与花菁5.5(Cy5.5)偶联而制成的。正如预期的那样,Cy5.5–MSA–G250 NP可以通过PTT在体内外有效杀死癌细胞。同时,我们意外地发现Cy5.5–MSA–G250对肿瘤细胞具有固有的特异性细胞毒性,但对正常细胞却没有。而且,Cy5.5–MSA–G250的肿瘤特异性细胞毒性要比FDA批准的抗癌药物阿糖胞苷强得多。体内实验还证明,Cy5.5–MSA–G250 NP可以有效消除残留的肿瘤细胞并预防转移。进一步的研究表明,在肿瘤细胞中选择性诱导G1细胞周期阻滞和抑制DNA复制可能是Cy5.5–MSA–G250 NPs对肿瘤细胞产生选择性细胞毒性的可能机制。此外,直接可视化,低系统毒性,良好的生物降解性和有效的人体排泄进一步使Cy5.5–MSA–G250 NP在体内应用中具有吸引力。总之,Cy5.5–MSA–G250 NP被证明是光热联合化疗的有前途的平台。进一步的研究表明,在肿瘤细胞中选择性诱导G1细胞周期阻滞和抑制DNA复制可能是Cy5.5–MSA–G250 NPs对肿瘤细胞产生选择性细胞毒性的可能机制。此外,直接可视化,低系统毒性,良好的生物降解性和有效的人体排泄进一步使Cy5.5–MSA–G250 NP在体内应用中具有吸引力。总之,Cy5.5–MSA–G250 NP被证明是光热联合化疗的有前途的平台。进一步的研究表明,在肿瘤细胞中选择性诱导G1细胞周期阻滞和抑制DNA复制可能是Cy5.5–MSA–G250 NPs对肿瘤细胞产生选择性细胞毒性的可能机制。此外,直接可视化,低系统毒性,良好的生物降解性和有效的人体排泄进一步使Cy5.5–MSA–G250 NP在体内应用中具有吸引力。总之,Cy5.5–MSA–G250 NP被证明是光热联合化疗的有前途的平台。
更新日期:2018-07-13
中文翻译:

有机光热纳米剂的内在癌细胞选择性毒性:癌症联合光热化学疗法的简单配方
纳米药物介导的光热疗法(PTT)与化学疗法相结合已被提出作为一种有效的抗癌策略。但是,化学治疗剂通常会引起严重的副作用。在本文中,开发了一种新型PTT纳米剂(Cy5.5–MSA–G250),具有预料不到的内在肿瘤选择性细胞毒性。Cy5.5–MSA–G250纳米颗粒(NPs)是通过将小鼠血清白蛋白(MSA)和考马斯亮蓝(G250)混合,然后与花菁5.5(Cy5.5)偶联而制成的。正如预期的那样,Cy5.5–MSA–G250 NP可以通过PTT在体内外有效杀死癌细胞。同时,我们意外地发现Cy5.5–MSA–G250对肿瘤细胞具有固有的特异性细胞毒性,但对正常细胞却没有。而且,Cy5.5–MSA–G250的肿瘤特异性细胞毒性要比FDA批准的抗癌药物阿糖胞苷强得多。体内实验还证明,Cy5.5–MSA–G250 NP可以有效消除残留的肿瘤细胞并预防转移。进一步的研究表明,在肿瘤细胞中选择性诱导G1细胞周期阻滞和抑制DNA复制可能是Cy5.5–MSA–G250 NPs对肿瘤细胞产生选择性细胞毒性的可能机制。此外,直接可视化,低系统毒性,良好的生物降解性和有效的人体排泄进一步使Cy5.5–MSA–G250 NP在体内应用中具有吸引力。总之,Cy5.5–MSA–G250 NP被证明是光热联合化疗的有前途的平台。进一步的研究表明,在肿瘤细胞中选择性诱导G1细胞周期阻滞和抑制DNA复制可能是Cy5.5–MSA–G250 NPs对肿瘤细胞产生选择性细胞毒性的可能机制。此外,直接可视化,低系统毒性,良好的生物降解性和有效的人体排泄进一步使Cy5.5–MSA–G250 NP在体内应用中具有吸引力。总之,Cy5.5–MSA–G250 NP被证明是光热联合化疗的有前途的平台。进一步的研究表明,在肿瘤细胞中选择性诱导G1细胞周期阻滞和抑制DNA复制可能是Cy5.5–MSA–G250 NPs对肿瘤细胞产生选择性细胞毒性的可能机制。此外,直接可视化,低系统毒性,良好的生物降解性和有效的人体排泄进一步使Cy5.5–MSA–G250 NP在体内应用中具有吸引力。总之,Cy5.5–MSA–G250 NP被证明是光热联合化疗的有前途的平台。































 京公网安备 11010802027423号
京公网安备 11010802027423号