当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental and theoretical investigation into visible-light-promoted selective hydrogenation of crotonaldehyde to crotonyl alcohol using Au–Co,Ni alloy nanoparticle supported layered double hydroxides†
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2018-07-13 00:00:00 , DOI: 10.1039/c8ta05383a Guanhua Zhang 1, 2, 3, 4, 5 , Xiaofeng Zhang 1, 2, 3, 4, 5 , Yue Meng 6, 7, 8, 9 , Xiaobo Zhou 10, 11, 12 , Guoxiang Pan 6, 7, 8, 9 , Zheming Ni 1, 2, 3, 4, 5 , Shengjie Xia 1, 2, 3, 4, 5
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2018-07-13 00:00:00 , DOI: 10.1039/c8ta05383a Guanhua Zhang 1, 2, 3, 4, 5 , Xiaofeng Zhang 1, 2, 3, 4, 5 , Yue Meng 6, 7, 8, 9 , Xiaobo Zhou 10, 11, 12 , Guoxiang Pan 6, 7, 8, 9 , Zheming Ni 1, 2, 3, 4, 5 , Shengjie Xia 1, 2, 3, 4, 5
Affiliation
|
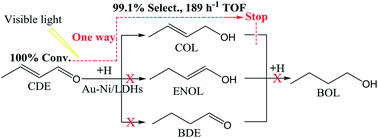
In this paper, Au–Co/Ni bimetal supported layered double hydroxides (Au–Co,Ni/LDHs) were synthesized for the hydrogenation reaction of crotonaldehyde (CDE) to crotonyl alcohol (COL) with both high conversion and selectivity under light irradiation. The hydrogenation experimental results indicated that both the hydrogenation conversion of CDE and the selectivity of COL have been greatly improved by Au–Co,Ni/LDHs under visible light irradiation, with the best turnover frequency performance of 189 h−1, 100% conversion and 99.1% selectivity (using Au–Ni/LDHs). Such high properties are attributed to a synergistic effect of localized surface plasmon resonance (LSPR) of Au nanoparticles, and the additional Co or Ni species would further enhance this effect. The DFT calculation of CDE hydrogenation at Au–Co,Ni/LDHs showed that the hydrogenation happens at C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O1 first to produce COL by following mechanism A2. Au–Ni/LDHs with lower activation energy had higher catalytic activity than Au–Co/LDHs. Further calculation showed that the COL hydration reaction can be hindered on Au–Co,Ni/LDHs compared to the unmodified Au/LDHs, which indicates that the introduction of second metal Ni or Co besides Au could further increase the COL productivity. The DFT calculations well explained and supported the experimental results.
O1 first to produce COL by following mechanism A2. Au–Ni/LDHs with lower activation energy had higher catalytic activity than Au–Co/LDHs. Further calculation showed that the COL hydration reaction can be hindered on Au–Co,Ni/LDHs compared to the unmodified Au/LDHs, which indicates that the introduction of second metal Ni or Co besides Au could further increase the COL productivity. The DFT calculations well explained and supported the experimental results.
中文翻译:

Au-Co,Ni合金纳米粒子担载的层状双氢氧化物对可见光促进巴豆醛选择性加氢成巴豆醇的实验和理论研究†
在本文中,合成了Au-Co / Ni双金属负载层状双氢氧化物(Au-Co,Ni / LDHs),用于巴豆醛(CDE)到巴豆醇(COL)的氢化反应,在光照射下具有高转化率和选择性。氢化实验结果表明,Au–Co,Ni / LDHs在可见光照射下,CDE的氢化转化率和COL的选择性均得到了极大的改善,其最佳的周转频率为189 h -1,100%的转化率和99.1%的选择性(使用Au-Ni / LDHs)。如此高的性能归因于Au纳米粒子的局部表面等离振子共振(LSPR)的协同效应,而其他Co或Ni物种将进一步增强这种效应。DFT在Au–Co,Ni / LDHs处CDE加氢的DFT计算表明,加氢首先发生在C2![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) O1处,并通过机理A2产生COL。具有较低活化能的Au-Ni / LDHs具有比Au-Co / LDHs更高的催化活性。进一步的计算表明,与未改性的Au / LDHs相比,Au-Co,Ni / LDHs可以阻止COL水合反应,这表明除Au之外引入第二种金属Ni或Co可以进一步提高COL生产率。DFT计算很好地解释了实验结果并提供了支持。
O1处,并通过机理A2产生COL。具有较低活化能的Au-Ni / LDHs具有比Au-Co / LDHs更高的催化活性。进一步的计算表明,与未改性的Au / LDHs相比,Au-Co,Ni / LDHs可以阻止COL水合反应,这表明除Au之外引入第二种金属Ni或Co可以进一步提高COL生产率。DFT计算很好地解释了实验结果并提供了支持。
更新日期:2018-07-13
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O1 first to produce COL by following mechanism A2. Au–Ni/LDHs with lower activation energy had higher catalytic activity than Au–Co/LDHs. Further calculation showed that the COL hydration reaction can be hindered on Au–Co,Ni/LDHs compared to the unmodified Au/LDHs, which indicates that the introduction of second metal Ni or Co besides Au could further increase the COL productivity. The DFT calculations well explained and supported the experimental results.
O1 first to produce COL by following mechanism A2. Au–Ni/LDHs with lower activation energy had higher catalytic activity than Au–Co/LDHs. Further calculation showed that the COL hydration reaction can be hindered on Au–Co,Ni/LDHs compared to the unmodified Au/LDHs, which indicates that the introduction of second metal Ni or Co besides Au could further increase the COL productivity. The DFT calculations well explained and supported the experimental results.
中文翻译:

Au-Co,Ni合金纳米粒子担载的层状双氢氧化物对可见光促进巴豆醛选择性加氢成巴豆醇的实验和理论研究†
在本文中,合成了Au-Co / Ni双金属负载层状双氢氧化物(Au-Co,Ni / LDHs),用于巴豆醛(CDE)到巴豆醇(COL)的氢化反应,在光照射下具有高转化率和选择性。氢化实验结果表明,Au–Co,Ni / LDHs在可见光照射下,CDE的氢化转化率和COL的选择性均得到了极大的改善,其最佳的周转频率为189 h -1,100%的转化率和99.1%的选择性(使用Au-Ni / LDHs)。如此高的性能归因于Au纳米粒子的局部表面等离振子共振(LSPR)的协同效应,而其他Co或Ni物种将进一步增强这种效应。DFT在Au–Co,Ni / LDHs处CDE加氢的DFT计算表明,加氢首先发生在C2
![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) O1处,并通过机理A2产生COL。具有较低活化能的Au-Ni / LDHs具有比Au-Co / LDHs更高的催化活性。进一步的计算表明,与未改性的Au / LDHs相比,Au-Co,Ni / LDHs可以阻止COL水合反应,这表明除Au之外引入第二种金属Ni或Co可以进一步提高COL生产率。DFT计算很好地解释了实验结果并提供了支持。
O1处,并通过机理A2产生COL。具有较低活化能的Au-Ni / LDHs具有比Au-Co / LDHs更高的催化活性。进一步的计算表明,与未改性的Au / LDHs相比,Au-Co,Ni / LDHs可以阻止COL水合反应,这表明除Au之外引入第二种金属Ni或Co可以进一步提高COL生产率。DFT计算很好地解释了实验结果并提供了支持。

































 京公网安备 11010802027423号
京公网安备 11010802027423号