当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enzymatic Assemblies Disrupt Membrane and Target Endoplasmic Reticulum (ER) for Selective Cancer Cell Death
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-07-11 , DOI: 10.1021/jacs.8b04641 Zhaoqianqi Feng 1 , Huaimin Wang 1 , Shiyu Wang 2 , Qiang Zhang 3 , Xixiang Zhang 3 , Avital A Rodal 2 , Bing Xu 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-07-11 , DOI: 10.1021/jacs.8b04641 Zhaoqianqi Feng 1 , Huaimin Wang 1 , Shiyu Wang 2 , Qiang Zhang 3 , Xixiang Zhang 3 , Avital A Rodal 2 , Bing Xu 1
Affiliation
|
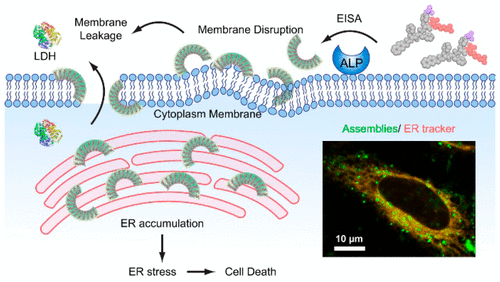
The endoplasmic reticulum (ER) is responsible for the synthesis and folding of a large number of proteins, as well as intracellular calcium regulation, lipid synthesis, and lipid transfer to other organelles, and is emerging as a target for cancer therapy. However, strategies for selectively targeting the ER of cancer cells are limited. Here we show that enzymatically generated crescent-shaped supramolecular assemblies of short peptides disrupt cell membranes and target ER for selective cancer cell death. As revealed by sedimentation assay, the assemblies interact with synthetic lipid membranes. Live cell imaging confirms that the assemblies impair membrane integrity, which is further supported by lactate dehydrogenase (LDH) assays. According to transmission electron microscopy (TEM), static light scattering (SLS), and critical micelle concentration (CMC), attaching an l-amino acid at the C-terminal of a d-tripeptide results in the crescent-shaped supramolecular assemblies. Structure-activity relationship suggests that the crescent-shaped morphology is critical for interacting with membranes and for controlling cell fate. Moreover, fluorescent imaging indicates that the assemblies accumulate on the ER. Time-dependent Western blot and ELISA indicate that the accumulation causes ER stress and subsequently activates the caspase signaling cascade for cell death. As an approach for in situ generating membrane binding scaffolds (i.e., the crescent-shaped supramolecular assemblies), this work promises a new way to disrupt the membrane and to target the ER for developing anticancer therapeutics.
中文翻译:

酶组装体破坏细胞膜并靶向内质网 (ER),从而实现选择性癌细胞死亡
内质网(ER)负责大量蛋白质的合成和折叠,以及细胞内钙调节、脂质合成和脂质转移到其他细胞器,并且正在成为癌症治疗的靶点。然而,选择性靶向癌细胞内质网的策略是有限的。在这里,我们展示了酶促生成的新月形短肽超分子组装体会破坏细胞膜并靶向内质网,从而选择性地杀死癌细胞。沉降测定表明,这些组件与合成脂质膜相互作用。活细胞成像证实这些组件会损害膜的完整性,乳酸脱氢酶 (LDH) 测定进一步支持了这一点。根据透射电子显微镜 (TEM)、静态光散射 (SLS) 和临界胶束浓度 (CMC),在 d-三肽的 C 端连接 L-氨基酸会产生新月形的超分子组装体。结构-活性关系表明新月形形态对于与膜相互作用和控制细胞命运至关重要。此外,荧光成像表明组装体聚集在内质网上。时间依赖性蛋白质印迹和 ELISA 表明,积累会导致 ER 应激,并随后激活 caspase 信号级联反应导致细胞死亡。作为一种原位生成膜结合支架(即新月形超分子组装体)的方法,这项工作有望提供一种破坏膜并靶向内质网以开发抗癌疗法的新方法。
更新日期:2018-07-11
中文翻译:

酶组装体破坏细胞膜并靶向内质网 (ER),从而实现选择性癌细胞死亡
内质网(ER)负责大量蛋白质的合成和折叠,以及细胞内钙调节、脂质合成和脂质转移到其他细胞器,并且正在成为癌症治疗的靶点。然而,选择性靶向癌细胞内质网的策略是有限的。在这里,我们展示了酶促生成的新月形短肽超分子组装体会破坏细胞膜并靶向内质网,从而选择性地杀死癌细胞。沉降测定表明,这些组件与合成脂质膜相互作用。活细胞成像证实这些组件会损害膜的完整性,乳酸脱氢酶 (LDH) 测定进一步支持了这一点。根据透射电子显微镜 (TEM)、静态光散射 (SLS) 和临界胶束浓度 (CMC),在 d-三肽的 C 端连接 L-氨基酸会产生新月形的超分子组装体。结构-活性关系表明新月形形态对于与膜相互作用和控制细胞命运至关重要。此外,荧光成像表明组装体聚集在内质网上。时间依赖性蛋白质印迹和 ELISA 表明,积累会导致 ER 应激,并随后激活 caspase 信号级联反应导致细胞死亡。作为一种原位生成膜结合支架(即新月形超分子组装体)的方法,这项工作有望提供一种破坏膜并靶向内质网以开发抗癌疗法的新方法。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号